Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D0PU8Z
|
||||
| Former ID |
DCL000511
|
||||
| Drug Name |
Dacetuzumab
|
||||
| Drug Type |
Monoclonal antibody
|
||||
| Indication | Large B-cell lymphoma; Non-hodgkin's lymphoma [ICD9: 202.8; ICD10:C81-C86] | Terminated | [537129] | ||
| Company |
Roche; Genentech
|
||||
| Structure |
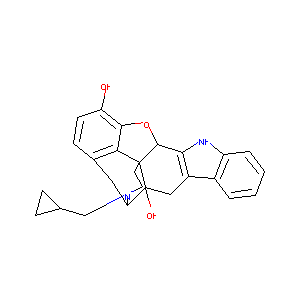
|
Download2D MOL |
|||
| Formula |
C26H29ClN2O4
|
||||
| PubChem Compound ID | |||||
| Target and Pathway | |||||
| Target(s) | Tumor necrosis factor ligand superfamily member 5 | Target Info | Modulator | ||
| KEGG Pathway | Cytokine-cytokine receptor interaction | ||||
| NF-kappa B signaling pathway | |||||
| Cell adhesion molecules (CAMs) | |||||
| T cell receptor signaling pathway | |||||
| Intestinal immune network for IgA production | |||||
| Malaria | |||||
| Toxoplasmosis | |||||
| Asthma | |||||
| Autoimmune thyroid disease | |||||
| Systemic lupus erythematosus | |||||
| Allograft rejection | |||||
| Primary immunodeficiency | |||||
| Viral myocarditis | |||||
| References | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

