Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D0O2WB
|
||||
| Former ID |
DAP000809
|
||||
| Drug Name |
Pyridostigmine
|
||||
| Synonyms |
Pyridostigminum; Regonol; Pyridostigmine Bromine; Mestinon (TN); Mestinon-SR; Regonol (TN); AQ-776/42801589; Pyridinium, 3-hydroxy-1-methyl-, dimethylcarbamate (ester); Pyridinium, 3-(((dimethylamino)carbonyl)oxy)-1-methyl-(9CI); (1-methylpyridin-1-ium-3-yl) N,N-dimethylcarbamate; 1-methyl-3-pyridiniumyl dimethylcarbamate; 3-(((Dimethylamino)carbonyl)oxy)-1-methylpyridinium
|
||||
| Drug Type |
Small molecular drug
|
||||
| Indication | Myasthenia gravis [ICD9: 358; ICD10:G70.0] | Approved | [1] | ||
| Therapeutic Class |
Parasympathomimetics
|
||||
| Company |
Valeant Pharmaceuticals
|
||||
| Structure |
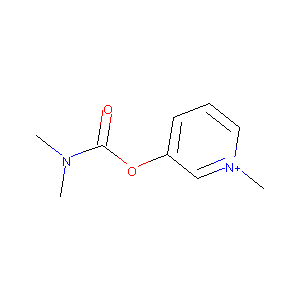
|
Download2D MOL |
|||
| Formula |
C9H13N2O2+
|
||||
| Canonical SMILES |
C[N+]1=CC=CC(=C1)OC(=O)N(C)C
|
||||
| InChI |
1S/C9H13N2O2/c1-10(2)9(12)13-8-5-4-6-11(3)7-8/h4-7H,1-3H3/q+1
|
||||
| InChIKey |
RVOLLAQWKVFTGE-UHFFFAOYSA-N
|
||||
| CAS Number |
CAS 155-97-5
|
||||
| PubChem Compound ID | |||||
| PubChem Substance ID |
9614, 866421, 4892591, 7437582, 7980440, 8153073, 11111694, 11335382, 11360621, 11364497, 11367059, 11369621, 11373077, 11377783, 11432882, 11461593, 11484637, 11488677, 11491724, 11495417, 15171335, 17153897, 29224064, 46506129, 47440132, 47662142, 47959602, 48334363, 50033601, 50100334, 50104217, 50361105, 90340824, 93166542, 95512043, 103310147, 103853547, 104147440, 104307965, 124750185, 124881247, 124881248, 124881249, 129188084, 134338090, 134974263, 137005304, 138967994, 140053019, 143458650
|
||||
| SuperDrug ATC ID |
N07AA02
|
||||
| SuperDrug CAS ID |
cas=000155975
|
||||
| Target and Pathway | |||||
| Target(s) | Acetylcholinesterase | Target Info | Inhibitor | [2] | |
| KEGG Pathway | Glycerophospholipid metabolism | ||||
| Cholinergic synapse | |||||
| PANTHER Pathway | Muscarinic acetylcholine receptor 1 and 3 signaling pathway | ||||
| Muscarinic acetylcholine receptor 2 and 4 signaling pathway | |||||
| Nicotinic acetylcholine receptor signaling pathway | |||||
| Pathway Interaction Database | ATF-2 transcription factor network | ||||
| PathWhiz Pathway | Phospholipid Biosynthesis | ||||
| WikiPathways | Monoamine Transport | ||||
| Biogenic Amine Synthesis | |||||
| Acetylcholine Synthesis | |||||
| Integrated Pancreatic Cancer Pathway | |||||
| References | |||||
| REF 1 | FDA Approved Drug Products from FDA Official Website. 2009. Application Number: (ANDA) 040457. | ||||
| REF 2 | Neuromuscular blockade, reversal agent use, and operating room time: retrospective analysis of US inpatient surgeries. Curr Med Res Opin. 2009 Apr;25(4):943-50. | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

