Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D0N1GH
|
||||
| Former ID |
DNC005719
|
||||
| Drug Name |
6-(pyridin-3-yl)-2-naphthonitrile
|
||||
| Drug Type |
Small molecular drug
|
||||
| Indication | Discovery agent | Investigative | [529834] | ||
| Structure |
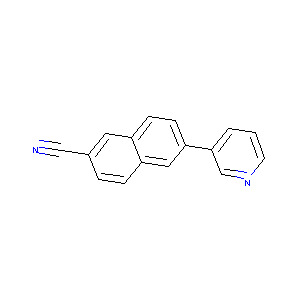
|
Download2D MOL |
|||
| Formula |
C16H10N2
|
||||
| Canonical SMILES |
C1=CC(=CN=C1)C2=CC3=C(C=C2)C=C(C=C3)C#N
|
||||
| InChI |
1S/C16H10N2/c17-10-12-3-4-14-9-15(6-5-13(14)8-12)16-2-1-7-18-11-16/h1-9,11H
|
||||
| InChIKey |
BRNSTMOAGOETDI-UHFFFAOYSA-N
|
||||
| PubChem Compound ID | |||||
| Target and Pathway | |||||
| Target(s) | Cytochrome P450 11B1, mitochondrial | Target Info | Inhibitor | [529624] | |
| 17 alpha-hydroxylase-C17, 20-lyase | Target Info | Inhibitor | [529834] | ||
| PathWhiz Pathway | SteroidogenesisPW000045:Androgen and Estrogen Metabolism | ||||
| Steroidogenesis | |||||
| WikiPathways | Metapathway biotransformation | ||||
| Oxidation by Cytochrome P450 | |||||
| Metabolism of steroid hormones and vitamin D | |||||
| Corticotropin-releasing hormoneWP702:Metapathway biotransformation | |||||
| Steroid Biosynthesis | |||||
| Glucocorticoid & Mineralcorticoid Metabolism | |||||
| Prostate Cancer | |||||
| Phase 1 - Functionalization of compounds | |||||
| References | |||||
| Ref 529624 | J Med Chem. 2008 Aug 28;51(16):5064-74. Epub 2008 Aug 1.Overcoming undesirable CYP1A2 inhibition of pyridylnaphthalene-type aldosterone synthase inhibitors: influence of heteroaryl derivatization onpotency and selectivity. | ||||
| Ref 529834 | J Med Chem. 2008 Dec 25;51(24):8077-87.In vivo active aldosterone synthase inhibitors with improved selectivity: lead optimization providing a series of pyridine substituted 3,4-dihydro-1H-quinolin-2-one derivatives. | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

