Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D0M9IC
|
||||
| Former ID |
DNC008643
|
||||
| Drug Name |
CONESSINE
|
||||
| Drug Type |
Small molecular drug
|
||||
| Indication | Discovery agent | Investigative | [1] | ||
| Structure |
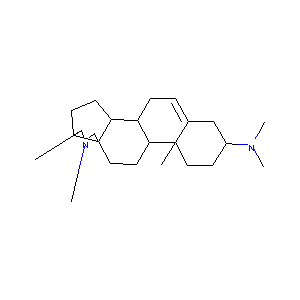
|
Download2D MOL |
|||
| Formula |
C24H40N2
|
||||
| Canonical SMILES |
CC1C2CCC3C2(CCC4C3CC=C5C4(CCC(C5)N(C)C)C)CN1C
|
||||
| InChI |
1S/C24H40N2/c1-16-20-8-9-22-19-7-6-17-14-18(25(3)4)10-12-23(17,2)21(19)11-13-24(20,22)15-26(16)5/h6,16,18-22H,7-15H2,1-5H3/t16-,18-,19+,20+,21-,22-,23-,24-/m0/s1
|
||||
| InChIKey |
GPLGAQQQNWMVMM-MYAJQUOBSA-N
|
||||
| PubChem Compound ID | |||||
| Target and Pathway | |||||
| Target(s) | Histamine H3 receptor | Target Info | Inhibitor | [2] | |
| Trypanothione reductase | Target Info | Inhibitor | [1] | ||
| Alpha-2C adrenergic receptor | Target Info | Inhibitor | [3] | ||
| KEGG Pathway | Neuroactive ligand-receptor interactionhsa04022:cGMP-PKG signaling pathway | ||||
| Neuroactive ligand-receptor interaction | |||||
| PANTHER Pathway | Alpha adrenergic receptor signaling pathway | ||||
| Heterotrimeric G-protein signaling pathway-Gi alpha and Gs alpha mediated pathway | |||||
| Reactome | Histamine receptors | ||||
| G alpha (i) signalling eventsR-HSA-390696:Adrenoceptors | |||||
| Adrenaline signalling through Alpha-2 adrenergic receptor | |||||
| Adrenaline,noradrenaline inhibits insulin secretion | |||||
| G alpha (i) signalling events | |||||
| G alpha (z) signalling events | |||||
| Surfactant metabolism | |||||
| WikiPathways | Monoamine Transport | ||||
| GPCRs, Class A Rhodopsin-like | |||||
| GPCR ligand binding | |||||
| GPCR downstream signalingWP58:Monoamine GPCRs | |||||
| Platelet Aggregation (Plug Formation) | |||||
| Integration of energy metabolism | |||||
| GPCR downstream signaling | |||||
| References | |||||
| REF 1 | Bioorg Med Chem. 2008 Jul 15;16(14):6689-95. Epub 2008 Jun 2.The use of natural product scaffolds as leads in the search for trypanothione reductase inhibitors. | ||||
| REF 2 | J Med Chem. 2009 Aug 13;52(15):4640-9.Design of a new histamine H3 receptor antagonist chemotype: (3aR,6aR)-5-alkyl-1-aryl-octahydropyrrolo[3,4-b]pyrroles, synthesis, and structure-activity relationships. | ||||
| REF 3 | J Med Chem. 2008 Sep 11;51(17):5423-30. Epub 2008 Aug 7.The alkaloid conessine and analogues as potent histamine H3 receptor antagonists. | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

