Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D0LR4B
|
||||
| Former ID |
DIB006702
|
||||
| Drug Name |
Loxoprofen gel
|
||||
| Synonyms |
Loxonin; Loxoprofen; Loxonin Pap; Loxonin Tape; CS-600G; LX-A; LX-P; Loxonin Gel 1%; Lipoxin A, Daiichi Sankyo/Lead Chemical; Lipoxin A, Sankyo/ Lead Chemical; Loxoprofen (gel, pain/ inflammation), Daiichi Sankyo; Loxoprofen (gel, pain/ inflammation), Sankyo; Loxoprofen (patch, pain/inflammation), Daiichi Sankyo/ Lead Chemical; Loxoprofen (patch, pain/inflammation), Sankyo/ Lead Chemical
|
||||
| Company |
Sankyo Co Ltd
|
||||
| Structure |
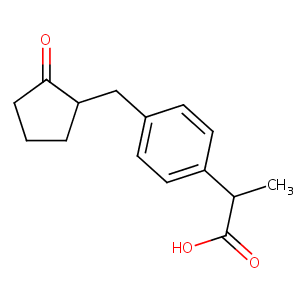
|
Download2D MOL |
|||
| Canonical SMILES |
C(=O)(C(c1ccc(CC2C(=O)CCC2)cc1)C)O
|
||||
| CAS Number |
CAS 68767-14-6
|
||||
| Target and Pathway | |||||
| Target(s) | Cyclooxygenase | Target Info | Inhibitor | [532557], [551871] | |
| References | |||||
| Ref 532557 | Comparative study of the clinical efficacy of the selective cyclooxygenase-2 inhibitor celecoxib compared with loxoprofen in patients with frozen shoulder. Mod Rheumatol. 2014 Jan;24(1):144-9. | ||||
| Ref 551871 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015 | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

