Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D0K5ER
|
||||
| Former ID |
DCL000633
|
||||
| Drug Name |
Rosiglitazone XR
|
||||
| Synonyms |
Avandia; Nyracta; Venvia; Rosiglitazone Maleate [USAN]; Rosiglitazone maleate; BRL 49653C; Avandia (TN); Avandiaadministration for 6-12 weeks; BRL 49653-C; BRL-49653C; SB-206846; SB-210232; Rosiglitazone maleate (JAN/USAN); (+-)-5-(p-(2-(Methyl-2-pyridylamino)ethoxy)benzyl)-2,4-thiazolidinedione maleate (1:1);(+-)-5-[[4-2-(methyl]-2-pyridinylamino)ethoxy]phenyl]methyl]-2,4-thiazolidinedione,(Z)-2-butenedioate (1:1); (Z)-but-2-enedioic acid; 2,4-Thiazolidinedione, 5-((4-(2-(methyl-2-pyridinylamino)ethoxy)phenyl)methyl)-, (2Z)-2-butenedioate (1:1); 2,4-Thiazolidinedione, 5-[[4-[2-(methyl-2-pyridinylamino) ethoxy]phenyl]methyl]-, (2Z)-2-butenedioate (1:1); 2,4-Thiazolidinedione,5-((4-(2-(methyl-2-pyridinylamino)ethoxy)phenyl)methyl)-,(2Z)-2-butenedioate; 5-[[4-[2-[methyl(pyridin-2-yl)amino]ethoxy]phenyl]methyl]-1,3-thiazolidine-2,4-dione; 5-{[4-({2-[methyl(pyridin-2-yl)amino]ethyl}oxy)phenyl]methyl}-1,3-thiazolidine-2,4-dione (2Z)-but-2-enedioate
|
||||
| Drug Type |
Small molecular drug
|
||||
| Company |
GSK
|
||||
| Structure |
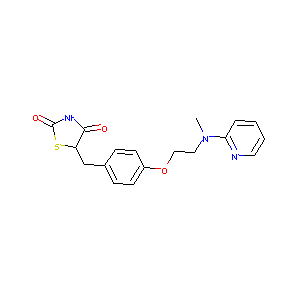
|
Download2D MOL |
|||
| Formula |
C22H23N3O7S
|
||||
| Canonical SMILES |
CN(CCOC1=CC=C(C=C1)CC2C(=O)NC(=O)S2)C3=CC=CC=N3.C(=CC(=<br />O)O)C(=O)O
|
||||
| InChI |
1S/C18H19N3O3S.C4H4O4/c1-21(16-4-2-3-9-19-16)10-11-24-14-7-5-13(6-8-14)12-15-17(22)20-18(23)25-15;5-3(6)1-2-4(7)8/h2-9,15H,10-12H2,1H3,(H,20,22,23);1-2H,(H,5,6)(H,7,8)/b;2-1-
|
||||
| InChIKey |
SUFUKZSWUHZXAV-BTJKTKAUSA-N
|
||||
| CAS Number |
CAS 122320-73-4
|
||||
| PubChem Compound ID | |||||
| PubChem Substance ID |
534302, 7847662, 8616490, 11528774, 12014878, 14932160, 25624753, 39289979, 46386628, 48416528, 49681563, 50085993, 50962349, 57357987, 91615938, 92308261, 92713029, 103221370, 104021279, 104253660, 113854462, 124658939, 124757841, 124800524, 125164645, 126625071, 126656372, 126670703, 131300304, 135035653, 136340308, 144212684, 152100953, 162037971, 164194065, 164196622, 172080316, 175607691, 179323691, 184553565, 186011363, 187072521, 196111584, 210275109, 210280748, 223378748, 223660290, 223775226, 225231964, 226408885
|
||||
| SuperDrug ATC ID |
A10BG02
|
||||
| SuperDrug CAS ID |
cas=122320734
|
||||
| Target and Pathway | |||||
| Target(s) | Peroxisome proliferator activated receptor gamma | Target Info | Agonist | [550963] | |
| PANTHER Pathway | CCKR signaling map ST | ||||
| WikiPathways | Wnt Signaling Pathway Netpath | ||||
| Nuclear Receptors in Lipid Metabolism and Toxicity | |||||
| Differentiation of white and brown adipocyte | |||||
| Regulation of Lipid Metabolism by Peroxisome proliferator-activated receptor alpha (PPARalpha) | |||||
| Transcriptional Regulation of White Adipocyte Differentiation | |||||
| Adipogenesis | |||||
| SREBP signalling | |||||
| Nuclear Receptors | |||||
| References | |||||
| Ref 531124 | Rosiglitazone monotherapy in mild-to-moderate Alzheimer's disease: results from a randomized, double-blind, placebo-controlled phase III study. Dement Geriatr Cogn Disord. 2010;30(2):131-46. | ||||
| Ref 550963 | Clinical pipeline report, company report or official report of GlaxoSmithKline (2009). | ||||
| Ref 556264 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

