Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D0K2HA
|
||||
| Former ID |
DIB004789
|
||||
| Drug Name |
PF-2413873
|
||||
| Synonyms |
PF-02413873
|
||||
| Drug Type |
Small molecular drug
|
||||
| Indication | Endometriosis [ICD9: 617; ICD10:N80] | Phase 1 | [1] | ||
| Company |
Pfizer Inc
|
||||
| Structure |
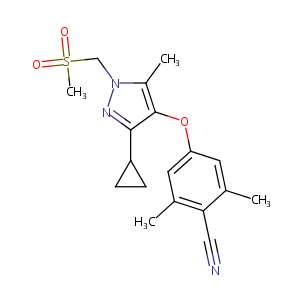
|
Download2D MOL |
|||
| Formula |
C18H21N3O3S
|
||||
| Canonical SMILES |
C(#N)c1c(cc(cc1C)Oc1c(nn(c1C)CS(=O)(=O)C)C1CC1)C
|
||||
| PubChem Compound ID | |||||
| Target and Pathway | |||||
| Target(s) | Progesterone receptor | Target Info | Antagonist | [2] | |
| KEGG Pathway | Oocyte meiosis | ||||
| Progesterone-mediated oocyte maturation | |||||
| Pathway Interaction Database | Cellular roles of Anthrax toxin | ||||
| Reactome | Nuclear signaling by ERBB4 | ||||
| Nuclear Receptor transcription pathway | |||||
| WikiPathways | Ovarian Infertility Genes | ||||
| Signaling by ERBB4 | |||||
| Nuclear Receptors | |||||
| References | |||||
| REF 1 | ClinicalTrials.gov (NCT00800618) A Study To Investigate How The Body Handles Multiple Doses Of PF-0243873 And To Investigate The Effect Of PF-02413873 On Sex Hormone Levels In Healthy Young Women. U.S. National Institutes of Health. | ||||
| REF 2 | Unusual base-catalyzed exchange in the synthesis of deuterated PF-2413873. Journal of Labelled Compounds. 08/2009; 52(10):435 - 442. | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

