Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D0I3YX
|
||||
| Former ID |
DNCL001889
|
||||
| Drug Name |
Capadenoson
|
||||
| Drug Type |
Small molecular drug
|
||||
| Indication | Atrial fibrillation [ICD9: 272, 427.31; ICD10:E78, I48] | Phase 2 | [1] | ||
| Company |
Bayer HealthCare Pharmaceuticals
|
||||
| Structure |
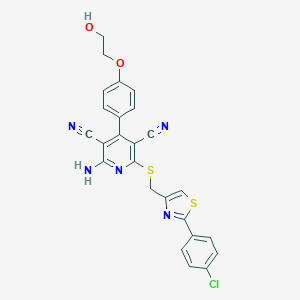
|
Download2D MOL |
|||
| Formula |
C25H18ClN5O2S2
|
||||
| PubChem Compound ID | |||||
| Target and Pathway | |||||
| Target(s) | Adenosine A1 receptor | Target Info | Agonist | [2] | |
| KEGG Pathway | cGMP-PKG signaling pathway | ||||
| cAMP signaling pathway | |||||
| Sphingolipid signaling pathway | |||||
| Neuroactive ligand-receptor interaction | |||||
| Morphine addiction | |||||
| NetPath Pathway | TCR Signaling Pathway | ||||
| RANKL Signaling Pathway | |||||
| PANTHER Pathway | Heterotrimeric G-protein signaling pathway-Gi alpha and Gs alpha mediated pathway | ||||
| Heterotrimeric G-protein signaling pathway-Gq alpha and Go alpha mediated pathway | |||||
| Reactome | Adenosine P1 receptors | ||||
| G alpha (i) signalling events | |||||
| WikiPathways | Nucleotide GPCRs | ||||
| GPCRs, Class A Rhodopsin-like | |||||
| GPCR ligand binding | |||||
| GPCR downstream signaling | |||||
| References | |||||
| REF 1 | ClinicalTrials.gov (NCT00568945) Study to Investigate the Effect of the A1 Agonist Capadenoson on Ventricular HR in Patients With Persistent or Permanent Atrial Fibrillation.. U.S. National Institutes of Health. | ||||
| REF 2 | A1 adenosine receptor agonists and their potential therapeutic applications. Expert Opin Investig Drugs. 2008 Dec;17(12):1901-10. | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

