| Drug General Information |
| Drug ID |
D0I1OS
|
| Former ID |
DIB009977
|
| Drug Name |
E-2212
|
| Indication |
Alzheimer disease [ICD9: 331; ICD10:G30]
|
Phase 1 |
[1]
|
|---|
| Company |
Eisai Co Ltd
|
| Structure |
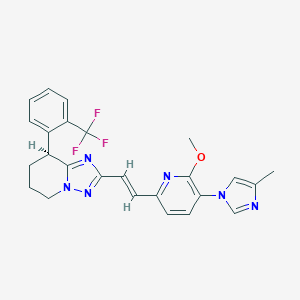
|
Download
2D MOL
3D MOL
|
| Target and Pathway |
| Target(s) |
Gamma-secretase |
Target Info |
Modulator |
[2]
|
|---|
|
KEGG Pathway
|
Notch signaling pathway
|
|
Alzheimer's disease
|
|
PANTHER Pathway
|
Alzheimer disease-amyloid secretase pathway
|
|
Alzheimer disease-presenilin pathway
|
|
Notch signaling pathway
|
|
Pathway Interaction Database
|
Notch signaling pathway
|
|
Presenilin action in Notch and Wnt signaling
|
|
p75(NTR)-mediated signaling
|
|
Syndecan-3-mediated signaling events
|
|
Reactome
|
Nuclear signaling by ERBB4
|
|
Regulated proteolysis of p75NTR
|
|
NRIF signals cell death from the nucleus
|
|
Activated NOTCH1 Transmits Signal to the Nucleus
|
|
Constitutive Signaling by NOTCH1 PEST Domain Mutants
|
|
Constitutive Signaling by NOTCH1 HD+PEST Domain Mutants
|
|
NOTCH2 Activation and Transmission of Signal to the Nucleus
|
|
EPH-ephrin mediated repulsion of cells
|
|
WikiPathways
|
Notch Signaling Pathway
|
|
Signaling by ERBB4
|
|
Signaling by NOTCH3
|
|
Signaling by NOTCH4
|
|
Signaling by NOTCH1
|
|
Signaling by NOTCH2
|
|
Notch Signaling Pathway
|
|
Signalling by NGF
|
| References |
| REF 1 | ClinicalTrials.gov (NCT01221259) A Randomized, Double-blind, Placebo-controlled, Sequential Ascending, Single-dose Study to Evaluate the Safety, Tolerability, Pharmacokinetics, and Pharmacodynamics of E2212 in Healthy Subjects. U.S. National Institutes of Health. |
|---|
| REF 2 | Development and mechanism of gamma-secretase modulators for Alzheimer's disease. Biochemistry. 2013 May 14;52(19):3197-216. |
|---|