Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D0H7XJ
|
||||
| Former ID |
DIB002791
|
||||
| Drug Name |
ASP-9521
|
||||
| Indication | Prostate cancer [ICD9: 185; ICD10:C61] | Phase 1/2 | [1] | ||
| Company |
Astellas Pharma Inc
|
||||
| Structure |
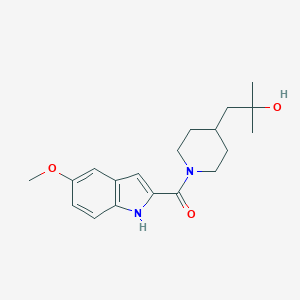
|
Download2D MOL |
|||
| Target and Pathway | |||||
| Target(s) | Aldo-keto reductase family 1 member C3 | Target Info | Modulator | [2], [3] | |
| BioCyc Pathway | Superpathway of steroid hormone biosynthesis | ||||
| Allopregnanolone biosynthesis | |||||
| Androgen biosynthesis | |||||
| KEGG Pathway | Steroid hormone biosynthesis | ||||
| Arachidonic acid metabolism | |||||
| Metabolic pathways | |||||
| Ovarian steroidogenesis | |||||
| NetPath Pathway | TGF_beta_Receptor Signaling Pathway | ||||
| PathWhiz Pathway | Arachidonic Acid Metabolism | ||||
| Reactome | Retinoid metabolism and transport | ||||
| WikiPathways | Metapathway biotransformation | ||||
| Benzo(a)pyrene metabolism | |||||
| Arachidonic acid metabolism | |||||
| References | |||||
| REF 1 | ClinicalTrials.gov (NCT01352208) Phase I/II Study of ASP9521 in Castrate-Resistant Prostate Cancer (CRPC) Patients. U.S. National Institutes of Health. | ||||
| REF 2 | Safety, tolerability and anti-tumour activity of the androgen biosynthesis inhibitor ASP9521 in patients with metastatic castration-resistant prostate cancer: multi-centre phase I/II study. Invest New Drugs. 2014 Oct;32(5):995-1004. | ||||
| REF 3 | In vitro and in vivo characterisation of ASP9521: a novel, selective, orally bioavailable inhibitor of 17beta-hydroxysteroid dehydrogenase type 5 (17betaHSD5; AKR1C3). Invest New Drugs. 2014 Oct;32(5):860-70. | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

