Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D0CQ6I
|
||||
| Former ID |
DNC006112
|
||||
| Drug Name |
3-[(2-methyl-4-thiazolyl)ethynyl]-5-vinylpyridine
|
||||
| Drug Type |
Small molecular drug
|
||||
| Indication | Discovery agent | Investigative | [1] | ||
| Structure |
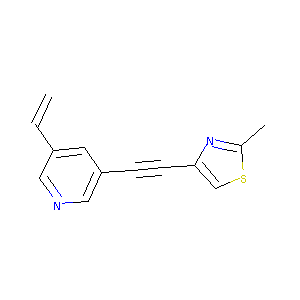
|
Download2D MOL |
|||
| Formula |
C13H10N2S
|
||||
| Canonical SMILES |
CC1=NC(=CS1)C#CC2=CC(=CN=C2)C=C
|
||||
| InChI |
1S/C13H10N2S/c1-3-11-6-12(8-14-7-11)4-5-13-9-16-10(2)15-13/h3,6-9H,1H2,2H3
|
||||
| InChIKey |
BZJCMXLEGBDDGI-UHFFFAOYSA-N
|
||||
| PubChem Compound ID | |||||
| Target and Pathway | |||||
| Target(s) | Metabotropic glutamate receptor 5 | Target Info | Inhibitor | [1] | |
| KEGG Pathway | Calcium signaling pathway | ||||
| Neuroactive ligand-receptor interaction | |||||
| Gap junction | |||||
| Long-term potentiation | |||||
| Retrograde endocannabinoid signaling | |||||
| Glutamatergic synapse | |||||
| Huntington's disease | |||||
| PANTHER Pathway | Heterotrimeric G-protein signaling pathway-Gq alpha and Go alpha mediated pathway | ||||
| Metabotropic glutamate receptor group III pathway | |||||
| Metabotropic glutamate receptor group I pathway | |||||
| Endogenous cannabinoid signaling | |||||
| Reactome | G alpha (q) signalling events | ||||
| Class C/3 (Metabotropic glutamate/pheromone receptors) | |||||
| WikiPathways | Hypothetical Network for Drug Addiction | ||||
| GPCRs, Class C Metabotropic glutamate, pheromone | |||||
| Gastrin-CREB signalling pathway via PKC and MAPK | |||||
| GPCR ligand binding | |||||
| GPCR downstream signaling | |||||
| References | |||||
| REF 1 | J Med Chem. 2006 Feb 9;49(3):1080-100.Synthesis and structure-activity relationships of 3-[(2-methyl-1,3-thiazol-4-yl)ethynyl]pyridine analogues as potent, noncompetitive metabotropic glutamate receptor subtype 5 antagonists; search for cocaine medications. | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

