| Drug General Information |
| Drug ID |
D0C2ET
|
| Former ID |
DIB016716
|
| Drug Name |
(R)-mequitazine (incontinence/respiratory disease), Pierre Fabre
|
| Synonyms |
V-0162; Muscarinic M1/M2/M3 antagonist (incontinence/respiratorydisease), Pierre Fabre; (R)-mequitazine
|
| Indication |
Respiratory disease [ICD10:J00-J99]
|
Phase 2 |
[1]
|
|---|
| Company |
Pierre Fabre SA
|
| Structure |
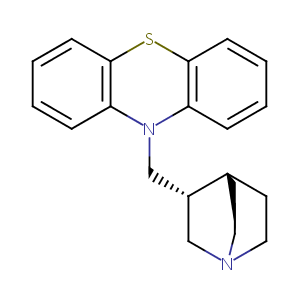
|
Download
2D MOL
3D MOL
|
| Canonical SMILES |
[C@H]1([C@@H]2CCN(C1)CC2)CN1c2ccccc2Sc2c1cccc2
|
| Target and Pathway |
| Target(s) |
Muscarinic acetylcholine receptor M3 |
Target Info |
Modulator |
|
|---|
|
KEGG Pathway
|
Calcium signaling pathway
|
|
Neuroactive ligand-receptor interaction
|
|
Cholinergic synapse
|
|
Regulation of actin cytoskeleton
|
|
Insulin secretion
|
|
Salivary secretion
|
|
Gastric acid secretion
|
|
Pancreatic secretion
|
|
PANTHER Pathway
|
Alzheimer disease-amyloid secretase pathway
|
|
Heterotrimeric G-protein signaling pathway-Gq alpha and Go alpha mediated pathway
|
|
Muscarinic acetylcholine receptor 1 and 3 signaling pathway
|
|
PathWhiz Pathway
|
Gastric Acid Production
|
|
Reactome
|
Muscarinic acetylcholine receptors
|
|
Acetylcholine regulates insulin secretion
|
|
G alpha (q) signalling events
|
|
WikiPathways
|
Monoamine GPCRs
|
|
Calcium Regulation in the Cardiac Cell
|
|
Regulation of Actin Cytoskeleton
|
|
GPCRs, Class A Rhodopsin-like
|
|
Gastrin-CREB signalling pathway via PKC and MAPK
|
|
Integration of energy metabolism
|
|
GPCR ligand binding
|
|
GPCR downstream signaling
|
|
GPCRs, Other
|
| References |
| REF 1 | ClinicalTrials.gov (NCT01951222) Bronchodilator Properties and Safety in Asthma. U.S. National Institutes of Health. |
|---|