Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D09PJX
|
||||
| Former ID |
DAP000756
|
||||
| Drug Name |
Tetrabenazine
|
||||
| Synonyms |
Nitoman; Rubigen; Tetrabenazina; Tetrabenazinum; Tetrabenzaine; Tetrabenzine; Xenazine; Tetra Benazin; Nitoman (TN); Ro 1-9569; Tetrabenazina [INN-Spanish]; Tetrabenazine (INN); Tetrabenazine [INN:BAN]; Tetrabenazinum [INN-Latin]; Xenazine (TN); Nitoman, Xenazine, Tetrabenazine; Ro 1-9569/12; 1,3,4,6,7,11b-Hexahydro-3-isobutyl-9,10-dimethoxy-2H-benzo(a)quinolizin-2-one; 1,3,4,6,7,11b-Hexahydro-3-isobutyl-9,10-dimethoxy-2H-benzo[a]quinolizin-2-one; 1,3,4,6,7,11b-hexahydro-9,10-dimethoxy-3-(2-methylpropyl)-2H-Benzo[a]quinolizin-2-one; 2-Oxo-3-isobutyl-9,10-dimethoxy-1,2,3,4,6,7-hexahydro-11bH-benzo[a]quinolizine; 2-Oxo-3-isobutyl-9,10-dimethoxy-1,3,4,6,7,11-beta-hexahydro-2H-benzoquinolizine; 2-Oxo-3-isobutyl-9,10-dimethoxy-1,3,4,6,7,11.beta.-hexahydro-2H-benzoquinolizine; 2H-Benzo(a)quinolizin-2-one, 1,3,4,6,7,11b-hexahydro-9,10-dimethoxy-3-(2-methylpropyl)-(9CI); 9,10-dimethoxy-3-(2-methylpropyl)-1,3,4,6,7,11b-hexahydro-2H-pyrido[2,1-a]isoquinolin-2-one; 9,10-dimethoxy-3-(2-methylpropyl)-1,3,4,6,7,11b-hexahydrobenzo[a]quinolizin-2-one
|
||||
| Drug Type |
Small molecular drug
|
||||
| Therapeutic Class |
Nephropathic cystinosis therapy
|
||||
| Structure |
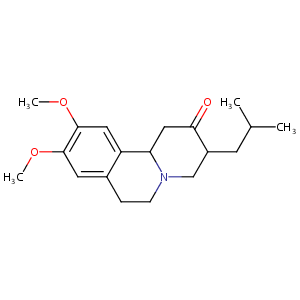
|
Download2D MOL |
|||
| Formula |
C19H27NO3
|
||||
| InChI |
InChI=1S/C19H27NO3/c1-12(2)7-14-11-20-6-5-13-8-18(22-3)19(23-4)9-15(13)16(20)10-17(14)21/h8-9,12,14,16H,5-7,10-11H2,1-4H3
|
||||
| InChIKey |
MKJIEFSOBYUXJB-UHFFFAOYSA-N
|
||||
| CAS Number |
CAS 58-46-8
|
||||
| PubChem Compound ID | |||||
| PubChem Substance ID |
13350, 442479, 444004, 5761629, 7914317, 7980763, 8153747, 10528302, 11501999, 14975282, 29216102, 29225032, 46506426, 47331537, 49834869, 49890235, 50086515, 51230337, 56311658, 57323119, 81066398, 85824684, 90341774, 92291104, 96025259, 99358095, 103928969, 104234203, 104253381, 104310884, 106228516, 117913352, 118252979, 121361938, 124530403, 124671347, 124757499, 125164303, 125205323, 125488233, 126658298, 126687256, 128608402, 134222085, 134338041, 135588520, 135610686, 135692279, 137248553, 141463478
|
||||
| SuperDrug ATC ID |
N07XX06
|
||||
| SuperDrug CAS ID |
cas=000058468
|
||||
| Target and Pathway | |||||
| Target(s) | Synaptic vesicle amine transporter | Target Info | Blocker | [537083], [537569] | |
| PANTHER Pathway | Adrenaline and noradrenaline biosynthesis | ||||
| 5HT1 type receptor mediated signaling pathway | |||||
| 5HT2 type receptor mediated signaling pathway | |||||
| 5HT3 type receptor mediated signaling pathway | |||||
| 5HT4 type receptor mediated signaling pathway | |||||
| Dopamine receptor mediated signaling pathway | |||||
| Nicotine pharmacodynamics pathway | |||||
| CCKR signaling map ST | |||||
| References | |||||
| Ref 468067 | (http://www.guidetopharmacology.org/) Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 4834). | ||||
| Ref 529941 | 2008 FDA drug approvals. Nat Rev Drug Discov. 2009 Feb;8(2):93-6. | ||||
| Ref 530894 | Role of tetrabenazine for Huntington's disease-associated chorea. Ann Pharmacother. 2010 Jun;44(6):1080-9. | ||||
| Ref 532471 | The druggable genome: Evaluation of drug targets in clinical trials suggests major shifts in molecular class and indication. Annu Rev Pharmacol Toxicol. 2014;54:9-26. | ||||
| Ref 538577 | FDA Approved Drug Products from FDA Official Website. 2009. Application Number: (NDA) 021894. | ||||
| Ref 551871 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015 | ||||
| Ref 537083 | 11C-dihydrotetrabenazine PET of the pancreas in subjects with long-standing type 1 diabetes and in healthy controls. J Nucl Med. 2009 Mar;50(3):382-9. Epub 2009 Feb 17. | ||||
| Ref 537569 | Dopamine signaling is required for depolarization-induced slow current in cerebellar Purkinje cells. J Neurosci. 2009 Jul 1;29(26):8530-8. | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

