Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D09GPB
|
||||
| Former ID |
DCL000461
|
||||
| Drug Name |
AZD2066
|
||||
| Drug Type |
Small molecular drug
|
||||
| Indication | Major depressive disorder; GERD; Chronic neuropathic pain [ICD9: 296.2, 296.3, 338, 356.0, 356.8, 530,780; ICD10:F32, F33, G64, G90.0, K21, R52, G89] | Discontinued in Phase 2 | [548676] | ||
| Therapeutic Class |
Analgesics
|
||||
| Company |
AstraZeneca
|
||||
| Structure |
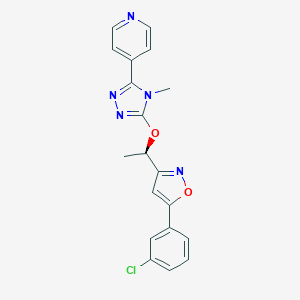
|
Download2D MOL |
|||
| Formula |
C19H16ClN5O2
|
||||
| PubChem Compound ID | |||||
| Target and Pathway | |||||
| Target(s) | Metabotropic glutamate receptor 5 | Target Info | Antagonist | [550288] | |
| References | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

