Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D09AOO
|
||||
| Former ID |
DCL001141
|
||||
| Drug Name |
ANAVEX 2-73
|
||||
| Indication | Breast cancer [ICD9: 174, 175; ICD10:C50] | Phase 2 | [1], [2] | ||
| Alzheimer disease [ICD9: 331; ICD10:G30] | Phase 1 | [3] | |||
| Company |
Anavex
|
||||
| Structure |
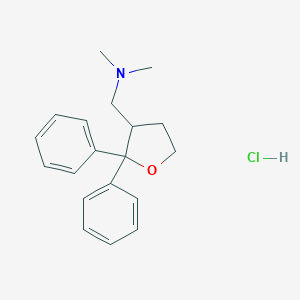
|
Download2D MOL |
|||
| Target and Pathway | |||||
| Target(s) | Sigma(1)-type opioid receptor | Target Info | Agonist | [3] | |
| WikiPathways | miR-targeted genes in squamous cell - TarBase | ||||
| miR-targeted genes in lymphocytes - TarBase | |||||
| References | |||||
| REF 1 | Anti-amnesic and neuroprotective potentials of the mixed muscarinic receptor/sigma 1 (?1) ligand ANAVEX2-73, a novel aminotetrahydrofuran derivative. J Psychopharmacol. 2011 Aug;25(8):1101-17. | ||||
| REF 2 | Blockade of Tau hyperphosphorylation and Abeta?????? generation by the aminotetrahydrofuran derivative ANAVEX2-73, a mixed muscarinic and ???receptor agonist, in a nontransgenic mouse model of Alzheimer's disease. Neuropsychopharmacology. 2013 Aug;38(9):1706-23. | ||||
| REF 3 | 2011 Pipeline of Anavex. | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

