Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D08MHD
|
||||
| Former ID |
DIB002510
|
||||
| Drug Name |
E-2609
|
||||
| Synonyms |
Beta secretase inhibitor (Alzheimer's disease), Eisai
|
||||
| Indication | Alzheimer disease [ICD9: 331; ICD10:G30] | Phase 2 | [525035] | ||
| Company |
Eisai Co Ltd
|
||||
| Structure |
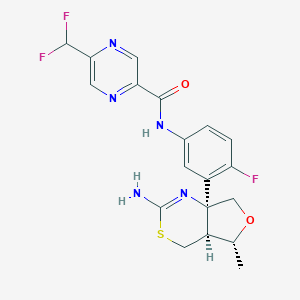
|
Download2D MOL |
|||
| Target and Pathway | |||||
| Target(s) | Beta-secretase | Target Info | Inhibitor | [544424] | |
| References | |||||
| Ref 525035 | ClinicalTrials.gov (NCT02322021) Dose-Finding Study To Evaluate Safety, Tolerability, and Efficacy of E2609 in Subjects With Mild Cognitive Impairment Due to Alzheimer's Disease (Prodromal Alzheimer's Disease) and Mild Dementia Due to Alzheimer's Disease. U.S. National Institutes of Health. | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

