Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D07XDQ
|
||||
| Former ID |
DIB008200
|
||||
| Drug Name |
Figopitant
|
||||
| Synonyms |
BIIF-1149; BIIF-1149CL; NK1 antagonists, Boehringer Ingelheim
|
||||
| Drug Type |
Small molecular drug
|
||||
| Indication | Diabetes [ICD9: 253.5, 588.1; ICD10:E23.2, N25.1] | Phase 1 | [1] | ||
| Company |
Boehringer Ingelheim Corp
|
||||
| Structure |
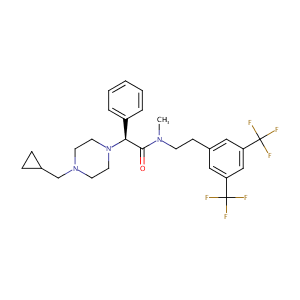
|
Download2D MOL |
|||
| Formula |
C27H31F6N3O
|
||||
| Canonical SMILES |
[C@H](N1CCN(CC1)CC1CC1)(c1ccccc1)C(=O)N(CCc1cc(cc(c1)C(<br />F)(F)F)C(F)(F)F)C
|
||||
| PubChem Compound ID | |||||
| Target and Pathway | |||||
| Target(s) | Substance-P receptor | Target Info | Antagonist | [2] | |
| KEGG Pathway | Calcium signaling pathway | ||||
| Neuroactive ligand-receptor interaction | |||||
| Measles | |||||
| PANTHER Pathway | CCKR signaling map ST | ||||
| Reactome | G alpha (q) signalling events | ||||
| WikiPathways | SIDS Susceptibility Pathways | ||||
| Gastrin-CREB signalling pathway via PKC and MAPK | |||||
| Spinal Cord Injury | |||||
| Peptide GPCRs | |||||
| GPCR ligand binding | |||||
| GPCR downstream signaling | |||||
| References | |||||
| REF 1 | ClinicalTrials.gov (NCT02199899) Safety, Tolerability and Pharmacodynamics of BIIF 1149 BS in Healthy Young Male Volunteers. U.S. National Institutes of Health. | ||||
| REF 2 | Investigation of figopitant and its metabolites in rat tissue by combining whole-body autoradiography with liquid extraction surface analysis mass spectrometry. Drug Metab Dispos. 2012 Mar;40(3):419-25. | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

