Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D06XWB
|
||||
| Former ID |
DNC000749
|
||||
| Drug Name |
Huperzine A
|
||||
| Drug Type |
Small molecular drug
|
||||
| Indication | Epileptic seizures; Alzheimer disease [ICD9: 345.9, 780.3, 331; ICD10:G40, P90, R56, G30] | Approved | [1], [2] | ||
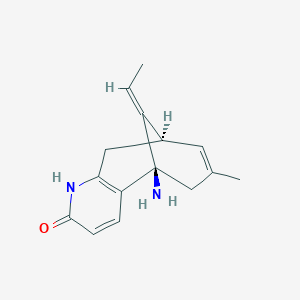
| Structure |
|
Download2D MOL |
|||
| Formula |
C15H18N2O
|
||||
| Canonical SMILES |
CC=C1C2CC3=C(C1(CC(=C2)C)N)C=CC(=O)N3
|
||||
| InChI |
1S/C15H18N2O/c1-3-11-10-6-9(2)8-15(11,16)12-4-5-14(18)17-13(12)7-10/h3-6,10H,7-8,16H2,1-2H3,(H,17,18)/b11-3+
|
||||
| InChIKey |
ZRJBHWIHUMBLCN-QDEBKDIKSA-N
|
||||
| CAS Number |
CAS 102518-79-6
|
||||
| PubChem Compound ID | |||||
| PubChem Substance ID |
24895708, 26756604, 29215281, 46511199, 46511231, 49991754, 49991755, 81041173, 85787773, 87462092, 91704460, 99301010, 99302530, 124892414, 134339656, 134339657, 134339658, 134340090, 135256104, 138769941, 142457861, 162093277, 162226717, 163395960, 163621066, 163686389, 163843547, 164758826, 180372184, 184548364, 216261296, 223435570, 226974040, 227296699, 242084598, 252455822
|
||||
| Target and Pathway | |||||
| Target(s) | Acetylcholinesterase | Target Info | Inhibitor | [3] | |
| NMDA receptor | Target Info | Antagonist | [4] | ||
| KEGG Pathway | Glycerophospholipid metabolism | ||||
| Cholinergic synapse | |||||
| PANTHER Pathway | Muscarinic acetylcholine receptor 1 and 3 signaling pathway | ||||
| Muscarinic acetylcholine receptor 2 and 4 signaling pathway | |||||
| Nicotinic acetylcholine receptor signaling pathway | |||||
| Pathway Interaction Database | ATF-2 transcription factor network | ||||
| PathWhiz Pathway | Phospholipid Biosynthesis | ||||
| WikiPathways | Monoamine Transport | ||||
| Biogenic Amine Synthesis | |||||
| Acetylcholine Synthesis | |||||
| Integrated Pancreatic Cancer Pathway | |||||
| References | |||||
| REF 1 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800000687) | ||||
| REF 2 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015 | ||||
| REF 3 | Huperzine A attenuates cognitive deficits and brain injury in neonatal rats after hypoxia-ischemia. Brain Res. 2002 Sep 13;949(1-2):162-70. | ||||
| REF 4 | Progress report on new antiepileptic drugs: a summary of the Ninth Eilat Conference (EILAT IX). Epilepsy Res. 2009 Jan;83(1):1-43. Epub 2008 Nov 12. | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

