Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D06WRF
|
||||
| Former ID |
DNCL002783
|
||||
| Drug Name |
KD019
|
||||
| Drug Type |
Small molecular drug
|
||||
| Indication | Late-stage non-small cell lung cancer [ICD9: 162, 203.0, 208.9; ICD10:C33-C34] | Phase 2 | [1] | ||
| Company |
Kadmon Pharmaceuticals
|
||||
| Structure |
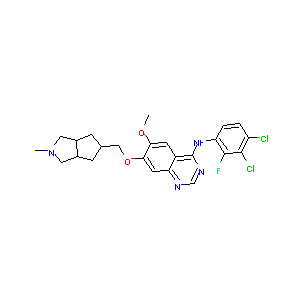
|
Download2D MOL |
|||
| Formula |
C24H25Cl2FN4O2
|
||||
| Canonical SMILES |
CN1CC2CC(CC2C1)COC3=C(C=C4C(=C3)N=CN=C4NC5=C(C(=C(C=C5)<br />Cl)Cl)F)OC
|
||||
| InChI |
1S/C24H25Cl2FN4O2/c1-31-9-14-5-13(6-15(14)10-31)11-33-21-8-19-16(7-20(21)32-2)24(29-12-28-19)30-18-4-3-17(25)22(26)23(18)27/h3-4,7-8,12-15H,5-6,9-11H2,1-2H3,(H,28,29,30)/t13?,14-,15+
|
||||
| InChIKey |
HVXKQKFEHMGHSL-GOOCMWNKSA-N
|
||||
| PubChem Compound ID | |||||
| PubChem Substance ID |
15480187, 22792072, 40585946, 76905083, 127347070, 135378337, 136340286, 136345873, 137747606, 137747791, 162011572, 162038219, 162109071, 163818983, 163819000, 164042005, 172919892, 184811990, 198983317, 223400036, 227010733, 227010734, 227010735, 243284522, 243309177, 249565627, 252225708, 252473918
|
||||
| Target and Pathway | |||||
| Target(s) | Ephrin type-B receptor 4 | Target Info | Modulator | [2], [3] | |
| KEGG Pathway | Axon guidance | ||||
| Pathway Interaction Database | EPHB forward signaling | ||||
| EphrinB-EPHB pathway | |||||
| Ephrin B reverse signaling | |||||
| Reactome | EPH-Ephrin signaling | ||||
| EPHB-mediated forward signaling | |||||
| EPH-ephrin mediated repulsion of cells | |||||
| WikiPathways | Vitamin D Receptor Pathway | ||||
| References | |||||
| REF 1 | ClinicalTrials.gov (NCT01487174) KD019 Versus Erlotinib in Subjects With Stage IIIB/IV Non Small Cell Lung Cancer With Progression After First- or Second-Line Chemotherapy. U.S. National Institutes of Health. | ||||
| REF 2 | XL647--a multitargeted tyrosine kinase inhibitor: results of a phase II study in subjects with non-small cell lung cancer who have progressed after responding to treatment with either gefitinib or erlotinib. J Thorac Oncol. 2012 Jan;7(1):219-26. | ||||
| REF 3 | Phase II study of the multitargeted tyrosine kinase inhibitor XL647 in patients with non-small-cell lung cancer. J Thorac Oncol. 2012 May;7(5):856-65. doi: 10.1097/JTO.0b013e31824c943f. | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

