Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D06FEA
|
||||
| Former ID |
DAP000360
|
||||
| Drug Name |
Dinoprostone
|
||||
| Synonyms |
Cervidil; Dinoproston; Dinoprostona; Dinoprostonum; Prepidil; Propess; Prostenon; Prostin; Prepidil Gel; Prostarmon E;Prostin E; Dinoprostone Prostaglandin E2; Minprositin E2; Minprostin E2; PGE2; PGE2 alpha; PGE2alpha; Prostaglandin E2; Prostaglandin E2 alpha; Prostaglandin E2alpha; Prostarmon E2; Prostin E2; U 12062; [3H]PGE2; Alpha, PGE2; Alpha, Prostaglandin E2; BML1-F07; Cervidil (TN); Dinoprostona [INN-Spanish]; Dinoprostone beta-Cyclodextrin Clathrate; Dinoprostonum [INN-Latin]; E2 alpha, Prostaglandin; E2, Prostaglandin; E2alpha, Prostaglandin; Gel, Prepidil; L-PGE2; L-Prostaglandin E2; Prepidil (TN); Propess (TN); Prostin E2 (TN); U-12062; BMS-279654 & PGE2; Dinoprostone (JAN/USP/INN); Dinoprostone [USAN:INN:BAN:JAN]; L-7-(3-Hydroxy-2-(3-hydroxy-1-octenyl)-5-oxocyclopentyl)-5-heptenoic acid; (15S)-Prostaglandin E2; (5Z,11-alpha,13E,15S)-11,15-Dihydroxy-9-oxoprosta-5,13-dien-1-oic acid; (5Z,11.alpha.,13E,15S)-11,15-Dihydroxy-9-oxoprosta-5,13-dien-1-oic acid; (5Z,11alpha,13E,15S)-11,15-Dihydroxy-9-oxoprosta-5,13-dien-1-oic; (5Z,11alpha,13E,15S)-11,15-Dihydroxy-9-oxoprosta-5,13-dienoic acid; (5Z,11alpha,13E,15S)-11,15-dihydroxy-9-oxoprosta-5,13-dien-1-oic acid; (5Z,13E)-(15S)-11alpha,15-Dihydroxy-9-oxoprost-13-enoate; (5Z,13E)-(15S)-11alpha,15-dihydroxy-9-oxoprosta-5,13-dienoate; (5Z,13E,15S)-11alpha,15-dihydroxy-9-oxoprosta-5,13-dien-1-oic acid; (E,Z)-(1R,2R,3R)-7-(3-Hydroxy-2-((3S)-(3-hydroxy-1-octenyl))-5-oxocyclopentyl)-5-heptenoic acid; (Z)-7-[(1R,2R,3R)-3-Hydroxy-2-[(E,3S)-3-hydroxyoct-1-enyl]-5-oxocyclopentyl]hept-5-enoic acid; 5-Heptenoic acid, 7-(3-hydroxy-2-(3-hydroxy-1-octenyl)-5-oxocyclopentyl)-(8CI); 7-(3-Hydroxy-2-(3-hydroxy-1-octenyl)-5-oxocyclopentyl)-5-heptenoic acid
|
||||
| Drug Type |
Small molecular drug
|
||||
| Therapeutic Class |
Prostaglandins
|
||||
| Company |
Pfizer Pharmaceuticals
|
||||
| Structure |
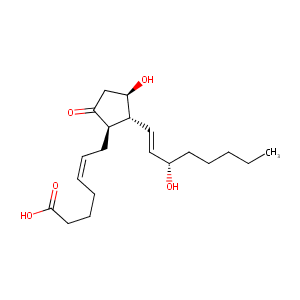
|
Download2D MOL |
|||
| Formula |
C20H32O5
|
||||
| InChI |
InChI=1S/C20H32O5/c1-2-3-6-9-15(21)12-13-17-16(18(22)14-19(17)23)10-7-4-5-8-11-20(24)25/h4,7,12-13,15-17,19,21,23H,2-3,5-6,8-11,14H2,1H3,(H,24,25)/b7-4-,13-12+/t15-,16+,17+,19+/m0/s1
|
||||
| InChIKey |
XEYBRNLFEZDVAW-ARSRFYASSA-N
|
||||
| CAS Number |
CAS 363-24-6
|
||||
| PubChem Compound ID | |||||
| PubChem Substance ID |
3863, 439749, 450259, 599053, 803734, 3139923, 3727087, 4265954, 7847147, 7979101, 8143217, 8616235, 10321741, 14778621, 14900948, 24898100, 24898683, 24898775, 26753268, 26753269, 26753270, 26759401, 39289526, 46505549, 47662021, 47810515, 47885164, 48415907, 50026789, 50087222, 50105678, 50105679, 53789659, 56313306, 56314112, 56459056, 57357707, 75054349, 85789494, 85856640, 92298396, 92308753, 92309906, 92722489, 99300820, 99302338, 99431525, 103176817, 104017641, 104046436
|
||||
| ChEBI ID |
ChEBI:15551
|
||||
| SuperDrug ATC ID |
G02AD02
|
||||
| SuperDrug CAS ID |
cas=000363246
|
||||
| Target and Pathway | |||||
| Target(s) | Prostaglandin E2receptor, EP2 subtype | Target Info | Antagonist | [537253] | |
| References | |||||
| Ref 538546 | FDA Approved Drug Products from FDA Official Website. 2009. Application Number: (NDA) 020411. | ||||
| Ref 539217 | (http://www.guidetopharmacology.org/) Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 1916). | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

