Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D05UHJ
|
||||
| Former ID |
DIB016307
|
||||
| Drug Name |
SM-10888
|
||||
| Indication | Cognitive disorders [ICD9: 290-294, 294.0, 780.09, 780.9, 780.93; ICD10:F01-F07, F04, F05, R41.3] | Discontinued in Phase 2 | [1] | ||
| Company |
Sumitomo Pharmaceuticals Co Ltd
|
||||
| Structure |
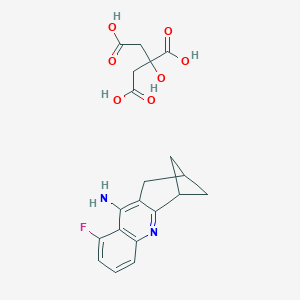
|
Download2D MOL |
|||
| Formula |
C54H55F3N6O14
|
||||
| Canonical SMILES |
c12c(nc3c(c1N)c(F)ccc3)C1CC(C2)C1.C(CC(=O)O)(CC(=O)O)(C<br />(=O)O)O
|
||||
| CAS Number |
CAS 116208-23-2
|
||||
| PubChem Compound ID | |||||
| Target and Pathway | |||||
| Target(s) | Acetylcholinesterase | Target Info | Inhibitor | [2] | |
| KEGG Pathway | Glycerophospholipid metabolism | ||||
| Cholinergic synapse | |||||
| PANTHER Pathway | Muscarinic acetylcholine receptor 1 and 3 signaling pathway | ||||
| Muscarinic acetylcholine receptor 2 and 4 signaling pathway | |||||
| Nicotinic acetylcholine receptor signaling pathway | |||||
| Pathway Interaction Database | ATF-2 transcription factor network | ||||
| PathWhiz Pathway | Phospholipid Biosynthesis | ||||
| WikiPathways | Monoamine Transport | ||||
| Biogenic Amine Synthesis | |||||
| Acetylcholine Synthesis | |||||
| Integrated Pancreatic Cancer Pathway | |||||
| References | |||||
| REF 1 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800001320) | ||||
| REF 2 | Pharmacological and biochemical assessment of SM-10888, a novel cholinesterase inhibitor. Jpn J Pharmacol. 1990 Jun;53(2):145-55. | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

