Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D05HSC
|
||||
| Former ID |
DAP000351
|
||||
| Drug Name |
Tubocurarine
|
||||
| Synonyms |
Amelizol; Delacurarine; Jexin; Tubadil; Tubaine; Tubarine; Tubocurarin; Tubocurarinum; Chlorure de tubocurarine; Cloruro de tubocurarina; Dextrotubocurarine chloride; Intocostrine T; Isoquinoline alkaloid; Tubocurarina cloruro; Tubocurarina cloruro [DCIT]; Tubocurarine chloride; Tubocurarine hydrochloride; Tubocurarini chloridum; Chlorure de tubocurarine [INN-French]; Cloruro de tubocurarina [INN-Spanish]; Curarin-haf; D-Paracurarine chloride; D-Tubocurarine; D-Tubocurarine chloride; D-Tubocurarine dichloride; D-Tubocurarine hydrochloride; Delacurarine (TN); Jex (TN); Metubine (TN); Tubaine (TN); Tubarine (TN); Tubocurarine chloride (INN); Tubocurarine chloride (TN); Tubocurarine chloride (anhydrous); Tubocurarini chloridum [INN-Latin]; Tubocurarinum (TN); D-(+)-Tubocurarine chloride; Tubocurarine, chloride, hydrochloride, (+)-(8CI); D-7',12'-Dihydroxy-6,6'-dimethoxy-2,2',2'-trimethyltubocuraranium chloride; Tubocuraranium, 7',12'-dihydroxy-6,6'-dimethoxy-2,2',2'-trimethyl-, chloride, hydrochloride; (+)-Tubocurarine; (+)-Tubocurarine chloride; (+)-Tubocurarine chloride hydrochloride; 2,2',2'-trimethyl-6,6'-bis(methyloxy)tubocuraran-2,2'-diium-7',12'-diol dichloride; 7',12'-Dihydroxy-6,6'-dimethoxy-2,2',2'-trimethyltubocuraranium; 7',12'-dihydroxy-6,6'-dimethoxy-2,2',2'-trimethyltubocuraran-2'-ium
|
||||
| Drug Type |
Small molecular drug
|
||||
| Therapeutic Class |
Neuromuscular Nondepolarizing Agents
|
||||
| Structure |
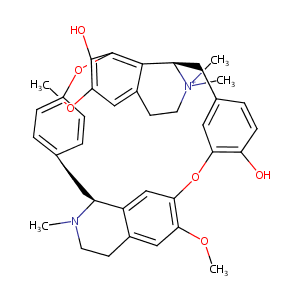
|
Download2D MOL |
|||
| Formula |
C37H41N2O6+
|
||||
| InChI |
InChI=1S/C37H40N2O6/c1-38-14-12-24-19-32(42-4)33-21-27(24)28(38)16-22-6-9-26(10-7-22)44-37-35-25(20-34(43-5)36(37)41)13-15-39(2,3)29(35)17-23-8-11-30(40)31(18-23)45-33/h6-11,18-21,28-29H,12-17H2,1-5H3,(H-,40,41)/p+1/t28-,29+/m0/s1
|
||||
| InChIKey |
JFJZZMVDLULRGK-URLMMPGGSA-O
|
||||
| CAS Number |
CAS 6989-98-6
|
||||
| PubChem Compound ID | |||||
| PubChem Substance ID |
9750, 598405, 7979097, 11341746, 11361929, 11364928, 11367490, 11370052, 11372089, 11375402, 11378222, 11485225, 11487331, 11489151, 11490910, 11493736, 11495780, 11537649, 15217798, 17425509, 26756599, 29225014, 46505279, 47736639, 47959927, 47959928, 48035288, 50070796, 50123130, 53789134, 57323106, 79261593, 92308722, 104037209, 104310830, 115001452, 115001455, 124892413, 134337662, 134453346, 134971353, 135652723, 135890516, 136948051, 137003129, 142970979, 160845901, 160964532, 164153589, 175443878
|
||||
| ChEBI ID |
ChEBI:9774
|
||||
| SuperDrug ATC ID |
M03AA02
|
||||
| SuperDrug CAS ID |
cas=000057954
|
||||
| Target and Pathway | |||||
| Target(s) | Neuronal acetylcholinereceptor subunit alpha-2 | Target Info | Antagonist | [536781], [537286] | |
| KEGG Pathway | Neuroactive ligand-receptor interaction | ||||
| PANTHER Pathway | Nicotinic acetylcholine receptor signaling pathway | ||||
| References | |||||
| Ref 538386 | FDA Approved Drug Products from FDA Official Website. 2009. Application Number: (NDA) 005657. | ||||
| Ref 539445 | (http://www.guidetopharmacology.org/) Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 2294). | ||||
| Ref 551871 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015 | ||||
| Ref 536781 | Synergy between pairs of competitive antagonists at adult human muscle acetylcholine receptors. Anesth Analg. 2008 Aug;107(2):525-33. | ||||
| Ref 537286 | Pharmacological characteristics of the inhibition of nondepolarizing neuromuscular blocking agents at human adult muscle nicotinic acetylcholine receptor. Anesthesiology. 2009 Jun;110(6):1244-52. | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

