Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D04SBH
|
||||
| Former ID |
DNC001531
|
||||
| Drug Name |
Zamifenacin
|
||||
| Drug Type |
Small molecular drug
|
||||
| Indication | Urinary incontinence [ICD9: 788.3; ICD10:N39.3, N39.4, R32] | Discontinued in Phase 3 | [1] | ||
| Structure |
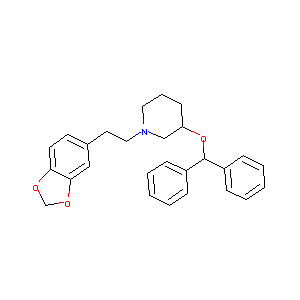
|
Download2D MOL |
|||
| Formula |
C27H29NO3
|
||||
| Canonical SMILES |
C1CC(CN(C1)CCC2=CC3=C(C=C2)OCO3)OC(C4=CC=CC=C4)C5=CC=CC<br />=C5
|
||||
| InChI |
1S/C27H29NO3/c1-3-8-22(9-4-1)27(23-10-5-2-6-11-23)31-24-12-7-16-28(19-24)17-15-21-13-14-25-26(18-21)30-20-29-25/h1-6,8-11,13-14,18,24,27H,7,12,15-17,19-20H2/t24-/m1/s1
|
||||
| InChIKey |
BDNFQGRSKSQXRI-XMMPIXPASA-N
|
||||
| CAS Number |
CAS 127308-82-1
|
||||
| PubChem Compound ID | |||||
| PubChem Substance ID | |||||
| Target and Pathway | |||||
| Target(s) | Muscarinic acetylcholine receptor M3 | Target Info | Antagonist | [2] | |
| KEGG Pathway | Calcium signaling pathway | ||||
| Neuroactive ligand-receptor interaction | |||||
| Cholinergic synapse | |||||
| Regulation of actin cytoskeleton | |||||
| Insulin secretion | |||||
| Salivary secretion | |||||
| Gastric acid secretion | |||||
| Pancreatic secretion | |||||
| PANTHER Pathway | Alzheimer disease-amyloid secretase pathway | ||||
| Heterotrimeric G-protein signaling pathway-Gq alpha and Go alpha mediated pathway | |||||
| Muscarinic acetylcholine receptor 1 and 3 signaling pathway | |||||
| PathWhiz Pathway | Gastric Acid Production | ||||
| Reactome | Muscarinic acetylcholine receptors | ||||
| Acetylcholine regulates insulin secretion | |||||
| G alpha (q) signalling events | |||||
| WikiPathways | Monoamine GPCRs | ||||
| Calcium Regulation in the Cardiac Cell | |||||
| Regulation of Actin Cytoskeleton | |||||
| GPCRs, Class A Rhodopsin-like | |||||
| Gastrin-CREB signalling pathway via PKC and MAPK | |||||
| Integration of energy metabolism | |||||
| GPCR ligand binding | |||||
| GPCR downstream signaling | |||||
| GPCRs, Other | |||||
| References | |||||
| REF 1 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800003650) | ||||
| REF 2 | Drug treatment options for irritable bowel syndrome: managing for success. Drugs Aging. 2001;18(3):201-11. | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

