Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D04QWQ
|
||||
| Former ID |
DNCL001683
|
||||
| Drug Name |
VX-509
|
||||
| Drug Type |
Small molecular drug
|
||||
| Indication | Rheumatoid arthritis [ICD9: 710-719, 714; ICD10:M05-M06] | Phase 2/3 | [1], [2] | ||
| Company |
Vertex Pharmaceuticals
|
||||
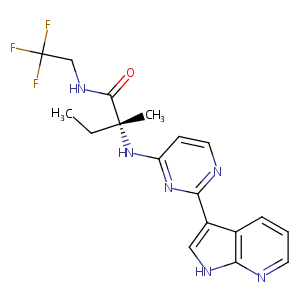
| Structure |
|
Download2D MOL |
|||
| Formula |
C18H19F3N6O
|
||||
| InChI |
InChI=1S/C18H19F3N6O/c1-3-17(2,16(28)25-10-18(19,20)21)27-13-6-8-23-15(26-13)12-9-24-14-11(12)5-4-7-22-14/h4-9H,3,10H2,1-2H3,(H,22,24)(H,25,28)(H,23,26,27)/t17-/m1/s1
|
||||
| InChIKey |
ASUGUQWIHMTFJL-QGZVFWFLSA-N
|
||||
| PubChem Compound ID | |||||
| PubChem Substance ID | |||||
| Target and Pathway | |||||
| Target(s) | Tyrosine-protein kinase JAK3 | Target Info | Inhibitor | [3], [4] | |
| KEGG Pathway | Chemokine signaling pathway | ||||
| PI3K-Akt signaling pathway | |||||
| Signaling pathways regulating pluripotency of stem cells | |||||
| Jak-STAT signaling pathway | |||||
| Measles | |||||
| HTLV-I infection | |||||
| Epstein-Barr virus infection | |||||
| Viral carcinogenesis | |||||
| Primary immunodeficiency | |||||
| PANTHER Pathway | Interleukin signaling pathway | ||||
| JAK/STAT signaling pathway | |||||
| PDGF signaling pathway | |||||
| Pathway Interaction Database | IL4-mediated signaling events | ||||
| CD40/CD40L signaling | |||||
| Signaling events mediated by TCPTP | |||||
| SHP2 signaling | |||||
| IL2-mediated signaling events | |||||
| IL2 signaling events mediated by PI3K | |||||
| IL2 signaling events mediated by STAT5 | |||||
| Reactome | GPVI-mediated activation cascade | ||||
| Interleukin-7 signaling | |||||
| G beta:gamma signalling through PI3Kgamma | |||||
| Interleukin-2 signaling | |||||
| RAF/MAP kinase cascade | |||||
| Interleukin receptor SHC signaling | |||||
| WikiPathways | IL-2 Signaling Pathway | ||||
| IL-4 Signaling Pathway | |||||
| Interleukin-2 signaling | |||||
| Interleukin-7 signaling | |||||
| Oncostatin M Signaling Pathway | |||||
| IL-9 Signaling Pathway | |||||
| IL-7 Signaling Pathway | |||||
| Interleukin-3, 5 and GM-CSF signaling | |||||
| References | |||||
| REF 1 | ClinicalTrials.gov (NCT01830985) A Phase 2/3 Open-label Extension Study to Evaluate Long-Term Safety and Efficacy With VX-509 in Subjects With Rheumatoid Arthritis. U.S. National Institutes of Health. | ||||
| REF 2 | (http://www.guidetopharmacology.org/) Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 8309). | ||||
| REF 3 | Selective JAK inhibitors in development for rheumatoid arthritis. Expert Opin Investig Drugs. 2014 Aug;23(8):1067-77. | ||||
| REF 4 | VX-509 (decernotinib) is a potent and selective janus kinase 3 inhibitor that attenuates inflammation in animal models of autoimmune disease. J Pharmacol Exp Ther. 2015 May;353(2):405-14. | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

