Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D03WQS
|
||||
| Former ID |
DCL000358
|
||||
| Drug Name |
BMS-582664
|
||||
| Synonyms |
Brivanib alaninate; BMS 582664; BMS582664; BMS-582664, Brivanib alaninate; Brivanib alaninate (INN/USAN); L-Alanine, (1R)-2-((4-((4-fluoro-2-methyl-1H-indol-5-yl)oxy)-5-methylpyrrolo(2,1-f)(1,2,4)triazin-6-yl)oxy)-1-methylethyl ester
|
||||
| Drug Type |
Small molecular drug
|
||||
| Indication | Hepatocellular carcinoma [ICD9: 155; ICD10:C22.0] | Phase 3 | [1], [2] | ||
| Therapeutic Class |
Anticancer Agents
|
||||
| Company |
Bristol Myers Squibb
|
||||
| Structure |
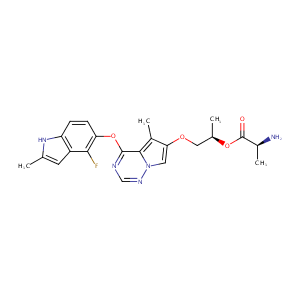
|
Download2D MOL |
|||
| Formula |
C22H24FN5O4
|
||||
| InChI |
InChI=1S/C22H24FN5O4/c1-11-7-15-16(27-11)5-6-17(19(15)23)32-21-20-13(3)18(8-28(20)26-10-25-21)30-9-12(2)31-22(29)14(4)24/h5-8,10,12,14,27H,9,24H2,1-4H3/t12-,14+/m1/s1
|
||||
| InChIKey |
LTEJRLHKIYCEOX-OCCSQVGLSA-N
|
||||
| CAS Number |
CAS 649735-63-7
|
||||
| PubChem Compound ID | |||||
| PubChem Substance ID |
16233765, 24458858, 42209717, 77346692, 92309407, 96025561, 99436997, 103577780, 124757026, 125163830, 126661322, 126680119, 126729215, 131480766, 135195809, 136340217, 136367326, 136920388, 142629726, 152258180, 160647016, 162011762, 162037460, 164837130, 174530181, 180423171, 184816560, 198987707, 223677760, 226799738, 248925490, 249565777, 252160520, 252438464, 252543406
|
||||
| Target and Pathway | |||||
| Target(s) | FGF-3 receptor | Target Info | Inhibitor | [3] | |
| KEGG Pathway | MAPK signaling pathway | ||||
| Ras signaling pathway | |||||
| Rap1 signaling pathway | |||||
| Endocytosis | |||||
| PI3K-Akt signaling pathway | |||||
| Signaling pathways regulating pluripotency of stem cells | |||||
| Regulation of actin cytoskeleton | |||||
| Pathways in cancer | |||||
| MicroRNAs in cancer | |||||
| Bladder cancer | |||||
| Central carbon metabolism in cancer | |||||
| PANTHER Pathway | FGF signaling pathway | ||||
| Reactome | FGFR3 mutant receptor activation | ||||
| WikiPathways | Regulation of Actin Cytoskeleton | ||||
| Endochondral Ossification | |||||
| Bladder Cancer | |||||
| Neural Crest Differentiation | |||||
| Signaling by FGFR | |||||
| References | |||||
| REF 1 | ClinicalTrials.gov (NCT00908752) Phase III Trans-Arterial Chemo-Embolization (TACE) Adjuvant HCC. U.S. National Institutes of Health. | ||||
| REF 2 | (http://www.guidetopharmacology.org/) Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 8097). | ||||
| REF 3 | A comparison of physicochemical property profiles of marketed oral drugs and orally bioavailable anti-cancer protein kinase inhibitors in clinical development. Curr Top Med Chem. 2007;7(14):1408-22. | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

