Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D03TGJ
|
||||
| Former ID |
DNAP001431
|
||||
| Drug Name |
Sorivudine
|
||||
| Synonyms |
Usevir (TN)
|
||||
| Drug Type |
Small molecular drug
|
||||
| Indication | Viral infections [ICD9: 054.0, 054.1, 054.2, 054.3, 075, 771.2, 052, 053; ICD10:B01, B02, A60, B00, B27, G05.1, P35.2] | Approved | [1] | ||
| Structure |
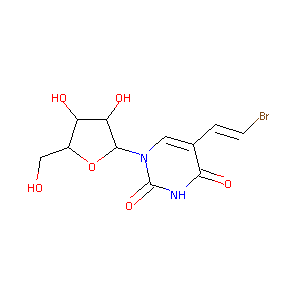
|
Download2D MOL |
|||
| Formula |
C11H13BrN2O6
|
||||
| Canonical SMILES |
C1=C(C(=O)NC(=O)N1C2C(C(C(O2)CO)O)O)C=CBr
|
||||
| InChI |
1S/C11H13BrN2O6/c12-2-1-5-3-14(11(19)13-9(5)18)10-8(17)7(16)6(4-15)20-10/h1-3,6-8,10,15-17H,4H2,(H,13,18,19)/b2-1+/t6-,7-,8+,10-/m1/s1
|
||||
| InChIKey |
GCQYYIHYQMVWLT-HQNLTJAPSA-N
|
||||
| CAS Number |
CAS 77181-69-2
|
||||
| PubChem Compound ID | |||||
| PubChem Substance ID | |||||
| SuperDrug ATC ID |
J05AB15
|
||||
| Target and Pathway | |||||
| Target(s) | HIV-1 reverse transcriptase | Target Info | Modulator | [2], [3] | |
| References | |||||
| REF 1 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015 | ||||
| REF 2 | New antivirals with activity against varicella-zoster virus. Ann Neurol. 1994;35 Suppl:S69-72. | ||||
| REF 3 | Natural products as sources of new drugs over the last 25 years. J Nat Prod. 2007 Mar;70(3):461-77. Epub 2007 Feb 20. | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

