Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D03RMN
|
||||
| Former ID |
DIB009612
|
||||
| Drug Name |
1069C
|
||||
| Drug Type |
Small molecular drug
|
||||
| Indication | Cancer [ICD9: 140-229; ICD10:C00-C96] | Phase 1 | [534331] | ||
| Structure |
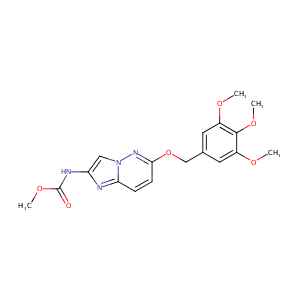
|
Download2D MOL |
|||
| Formula |
C18H20N4O6
|
||||
| Canonical SMILES |
COC1=CC(=CC(=C1OC)OC)COC2=NN3C=C(N=C3C=C2)NC(=O)OC
|
||||
| InChIKey |
UMSHZWFCVXIDEO-UHFFFAOYSA-N
|
||||
| PubChem Compound ID | |||||
| PubChem Substance ID | |||||
| Target and Pathway | |||||
| Target(s) | Tubulin beta | Target Info | Modulator | [550941], [551871] | |
| PANTHER Pathway | Cytoskeletal regulation by Rho GTPase | ||||
| Huntington disease | |||||
| References | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

