Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D03NHW
|
||||
| Former ID |
DAP000655
|
||||
| Drug Name |
Ofloxacin
|
||||
| Synonyms |
Bactocin; DEXTROFLOXACINE; Danoflox; Effexin; Exocin; Exocine; Flobacin; Flodemex; Flotavid; Flovid; Floxal; Floxil; Floxin; Floxstat; Fugacin; Inoflox; Kinflocin; Kinoxacin; Liflox; Loxinter; Marfloxacin; Medofloxine; Mergexin; Novecin; Nufafloqo; OFLX; OFX; Obide; Occidal; Ocuflox; Ofcin; Oflin; Oflocee; Oflocet; Oflocin; Oflodal; Oflodex; Oflodura; Oflox; Ofloxacina; Ofloxacine; Ofloxacino; Ofloxacinum; Ofloxin; Ofus; Onexacin; Operan; Orocin; Otonil; Oxaldin; Pharflox; Praxin; Puiritol; Qinolon; Qipro; Quinolon; Quotavil; Rilox; Sinflo; Tabrin; Taravid; Tariflox; Tarivid; Telbit; Tructum; Viotisone; Visiren; XED; Zanocin; Floxin otic; Ofloxacin Otic; Ofloxacina [DCIT]; Ofloxacine [French]; Ofloxacino [Spanish]; Ofloxacinum [Latin]; Uro Tarivid; DL 8280; HOE 280; O 8757; ORF 18489; PT 01; DL-8280; FLOXIN IN DEXTROSE 5%; FLOXIN IN DEXTROSE 5% IN PLASTIC CONTAINER; Floxin (TN); Floxin Otic (TN); HOE-280;Hoe-280; Marfloxacin (TN); O-Flox; ORF-28489; Ocuflox (TN); Ru-43280; WP-0405; Ofloxacin (JP15/USP/INN); Ofloxacin [USAN:BAN:INN:JAN]; Ofloxacin, (S)-Isomer; DL-8280, HOE-280, Exocin, Flobacin, Floxin, Floxil, Monoflocet, Ofloxacin; (+-)-9-Fluoro-2,3-dihydro-3-methyl-10-(4-methyl-1-piperazinyl)-7-oxo-7H-pyrido(1,2,3-de)-1,4-benzoxazine-6-carboxylic acid; (+/-)-9-Fluoro-2,3-dihydro-3-methyl-10-(4-methyl-1-piperaz inyl)-7-oxo-7H-pyrido(1,2,3-de)-1,4-benzoxazine-6-carboxylic acid; (+/-)-9-Fluoro-2,3-dihydro-3-methyl-10-(4-methyl-1-piperazinyl)-7-oxo-7H-pyrido(1,2,3-de)-1,4-benzoxazine-6-carboxylic acid; (+/-)-9-Fluoro-2,3-dihydro-3-methyl-10-(4-methyl-1-piperazinyl)-7-oxo-7H-pyrido[1,2,3-de]-1,4-benzoxazine-6-carboxylic acid; (+/-)-Floxin; (-)-9-Fluoro-2,3-dihydro-3-methyl-10-(4-methyl-1-piperazinyl)-7-oxo-7H-pyrido(1,2,3-de)(1,4)benzoxazin-6-carbonsaeure; 9-fluoro-3-methyl-10-(4-methylpiperazin-1-yl)-7-oxo-2,3-dihydro-7H-[1,4]oxazino[2,3,4-ij]quinoline-6-carboxylic acid; OFX
|
||||
| Drug Type |
Small molecular drug
|
||||
| Indication | Bacterial infections [ICD9: 001-009, 010-018, 020-027, 030-041, 080-088, 090-099, 100-104; ICD10:A00-B99] | Approved | [536222] | ||
| Therapeutic Class |
Antibiotics
|
||||
| Company |
Janssen Pharmaceutica
|
||||
| Structure |
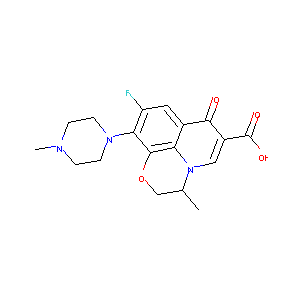
|
Download2D MOL |
|||
| Formula |
C18H20FN3O4
|
||||
| Canonical SMILES |
CC1COC2=C3N1C=C(C(=O)C3=CC(=C2N4CCN(CC4)C)F)C(=O)O
|
||||
| InChI |
1S/C18H20FN3O4/c1-10-9-26-17-14-11(16(23)12(18(24)25)8-22(10)14)7-13(19)15(17)21-5-3-20(2)4-6-21/h7-8,10H,3-6,9H2,1-2H3,(H,24,25)
|
||||
| InChIKey |
GSDSWSVVBLHKDQ-UHFFFAOYSA-N
|
||||
| CAS Number |
CAS 82419-36-1
|
||||
| PubChem Compound ID | |||||
| PubChem Substance ID |
9529, 597127, 3206370, 5025346, 7847519, 7891126, 7980182, 8027636, 8149812, 8152815, 11335896, 11361135, 11364080, 11366642, 11369204, 11372716, 11373806, 11377366, 11462107, 11466265, 11467385, 11485123, 11485970, 11489280, 11491314, 11492139, 11495000, 11533038, 12013736, 14876840, 17405462, 24278613, 24860450, 26612280, 26680409, 26747609, 26747610, 26747611, 26747612, 29223672, 46507574, 47216794, 47291150, 47515336, 47589018, 47589019, 47959764, 48035139, 48259249, 48259250
|
||||
| SuperDrug ATC ID |
J01MA01; S01AE01; S02AA16
|
||||
| SuperDrug CAS ID |
cas=082419361
|
||||
| Drug Resistance Mutation (DRM) | |||||
| DRM | DRM Info | ||||
| Target and Pathway | |||||
| Target(s) | Bacterial DNA gyrase | Target Info | Modulator | [556264] | |
| References | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

