Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D03GNI
|
||||
| Former ID |
DNC008784
|
||||
| Drug Name |
ISOQUINE
|
||||
| Drug Type |
Small molecular drug
|
||||
| Indication | Malaria [ICD10:B54] | Phase 1 | [1] | ||
| Structure |
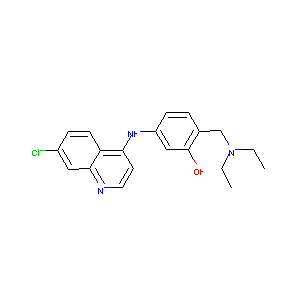
|
Download2D MOL |
|||
| Formula |
C20H22ClN3O
|
||||
| Canonical SMILES |
CCN(CC)CC1=C(C=C(C=C1)NC2=C3C=CC(=CC3=NC=C2)Cl)O
|
||||
| InChI |
1S/C20H22ClN3O/c1-3-24(4-2)13-14-5-7-16(12-20(14)25)23-18-9-10-22-19-11-15(21)6-8-17(18)19/h5-12,25H,3-4,13H2,1-2H3,(H,22,23)
|
||||
| InChIKey |
QGFYFOHMBDMGBZ-UHFFFAOYSA-N
|
||||
| PubChem Compound ID | |||||
| Target and Pathway | |||||
| Target(s) | Potassium voltage-gated channel subfamily H member 2 | Target Info | Inhibitor | [2] | |
| Cytochrome P450 2D6 | Target Info | Inhibitor | [2] | ||
| KEGG Pathway | Metabolism of xenobiotics by cytochrome P450 | ||||
| Drug metabolism - cytochrome P450 | |||||
| Serotonergic synapse | |||||
| PathWhiz Pathway | Muscle/Heart Contraction | ||||
| Reactome | Voltage gated Potassium channelsR-HSA-211981:Xenobiotics | ||||
| WikiPathways | SIDS Susceptibility Pathways | ||||
| Hematopoietic Stem Cell Differentiation | |||||
| Potassium ChannelsWP702:Metapathway biotransformation | |||||
| Tamoxifen metabolism | |||||
| Oxidation by Cytochrome P450 | |||||
| Vitamin D Receptor Pathway | |||||
| Aripiprazole Metabolic Pathway | |||||
| Fatty Acid Omega Oxidation | |||||
| Codeine and Morphine Metabolism | |||||
| References | |||||
| REF 1 | Antimalarial activity of isoquine against Kenyan Plasmodium falciparum clinical isolates and association with polymorphisms in pfcrt and pfmdr1 genes. J Antimicrob Chemother. 2013 Apr;68(4):786-8. | ||||
| REF 2 | J Med Chem. 2009 Mar 12;52(5):1408-15.Candidate selection and preclinical evaluation of N-tert-butyl isoquine (GSK369796), an affordable and effective 4-aminoquinoline antimalarial for the 21st century. | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

