Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D03CGQ
|
||||
| Former ID |
DIB004142
|
||||
| Drug Name |
AG-013958
|
||||
| Synonyms |
AG-13958
|
||||
| Indication | Age related macular degeneration [ICD9: 362.5; ICD10:H35.3] | Phase 1/2 | [1] | ||
| Company |
Pfizer Inc
|
||||
| Structure |
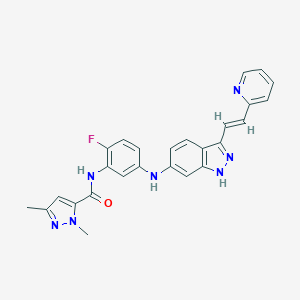
|
Download2D MOL |
|||
| Target and Pathway | |||||
| Target(s) | VEGF receptor | Target Info | Inhibitor | [2] | |
| KEGG Pathway | Ras signaling pathway | ||||
| Rap1 signaling pathway | |||||
| Cytokine-cytokine receptor interaction | |||||
| HIF-1 signaling pathway | |||||
| Endocytosis | |||||
| PI3K-Akt signaling pathway | |||||
| Focal adhesion | |||||
| Transcriptional misregulation in cancer | |||||
| Rheumatoid arthritis | |||||
| References | |||||
| REF 1 | ClinicalTrials.gov (NCT00090532) A Study Of The Safety And Efficacy Of AG-013,958 In Subjects With Subfoveal Choroidal Neovascularization Associated With Age-Related Macular Degeneration. U.S. National Institutes of Health. | ||||
| REF 2 | Receptor Tyrosine Kinase Inhibitors AG013764 and AG013711 Reduce Choroidal Neovascularization in Rat Eye. Exp Eye Res. 2007 May; 84(5): 922-933. | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

