Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D02NSF
|
||||
| Former ID |
DIB004983
|
||||
| Drug Name |
Oxymorphone
|
||||
| Synonyms |
Oxymorphone (abuse-resistant, pain); Oxymorphone (abuse-resistant, pain), Pain Therapeutics/ King Pharmaceuticals; Oxymorphone (abuse-resistant, pain), Pain Therapeutics/Pfizer
|
||||
| Drug Type |
Small molecular drug
|
||||
| Therapeutic Class |
Neurology Agents
|
||||
| Company |
Pain Therapeutics Inc
|
||||
| Structure |
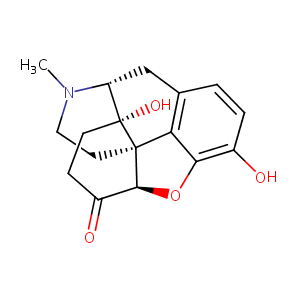
|
Download2D MOL |
|||
| Formula |
C17H19NO4
|
||||
| InChIKey |
UQCNKQCJZOAFTQ-ISWURRPUSA-N
|
||||
| PubChem Compound ID | |||||
| PubChem Substance ID |
10219, 81989, 841905, 11039374, 14751763, 14898432, 39317908, 46505296, 48416368, 50523562, 57359138, 92714667, 96025010, 103250876, 103916122, 118046192, 126629458, 129404545, 134223676, 134338383, 134971543, 137003774, 139076556, 152101651, 160964525, 162224633, 175267332, 178103672, 179117107, 210279774, 210282097, 223440572, 223822210, 226395721, 252355643
|
||||
| Target and Pathway | |||||
| Target(s) | Opioid receptor | Target Info | Modulator | [542100] | |
| References | |||||
| Ref 522753 | ClinicalTrials.gov (NCT00955110) Study to Compare Oxymorphone Extended-Release (Opana ER) Versus Oxycodone Controlled-Release (Oxycontin). U.S. National Institutes of Health. | ||||
| Ref 542100 | (http://www.guidetopharmacology.org/) Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7094). | ||||
| Ref 551871 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015 | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

