Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D02KRS
|
||||
| Former ID |
DAP000781
|
||||
| Drug Name |
Amantadine
|
||||
| Synonyms |
Adamantamine; Adamantanamine; Adamantylamine; Amant; Amantadina; Amantadinum; Amantidine; Aminoadamantane; Endantadine; Mantadine; Symadine; Symmetrel; Wiregyt; Amantadine Base; BIA4304; OR14310; Amantadina [INN-Spanish]; Amantadine (INN); Amantadine [INN:BAN]; Amantadinum [INN-Latin]; Gen-Amantadine; Pk-merz; Symmetrel (TN); TCMDC-125869; ADAMANTANE,1-AMINO; Adamantan-1-amine; Adamantan-1-ylamine; Tricyclo[3.3.1.1^3,7]decan-1-amine; Tricyclo(3.3.1.13,7)decan-1-amine; Tricyclo[3.3.1.1(3,7)]decan-1-amine;Tricyclo[3.3.1.1(3,7)]decan-1-ylamine; Tricyclo[3.3.1.1(3,7)]decane-1-amine; Tricyclo[3.3.1.1(sup3,7)]decan-1-amine; Tricyclo(3.3.1.1(sup 3,7))decan-1-amine; Tricyclo(3.3.1.1(sup 3.7))decan-1-amine; Tricyclo[3.3.1.1~3,7~]decan-1-amine; Tricyclo(3.3.1.1(3,7))-decan-1-amine; 1-Adamantamine; 1-Adamantanamine; 1-Adamantanamine (8CI); 1-Adamantylamine; 1-Aminoadamantane; 1-Aminotricyclo(3.3.1.1(sup 3,7))decane
|
||||
| Drug Type |
Small molecular drug
|
||||
| Therapeutic Class |
Antiviral Agents
|
||||
| Structure |
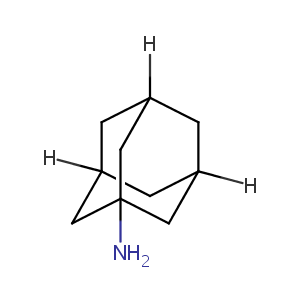
|
Download2D MOL |
|||
| Formula |
C10H17N
|
||||
| InChI |
InChI=1S/C10H17N/c11-10-4-7-1-8(5-10)3-9(2-7)6-10/h7-9H,1-6,11H2
|
||||
| InChIKey |
DKNWSYNQZKUICI-UHFFFAOYSA-N
|
||||
| CAS Number |
CAS 768-94-5
|
||||
| PubChem Compound ID | |||||
| PubChem Substance ID |
9036, 461565, 588114, 597379, 3136147, 5106490, 7978680, 8147192, 8151445, 10535392, 11110715, 11335990, 11361229, 11362804, 11365366, 11367928, 11371422, 11373687, 11376090, 11448576, 11462201, 11466435, 11467555, 11483738, 11486009, 11487897, 11490107, 11491949, 11493844, 15170902, 15219251, 16666659, 24848359, 26755640, 26755641, 29221309, 30400536, 38395099, 46168239, 46507081, 47216816, 47216817, 47440544, 47515356, 47589230, 47736526, 47959791, 48035167, 48035168, 48259495
|
||||
| ChEBI ID |
ChEBI:2618
|
||||
| SuperDrug ATC ID |
N04BB01
|
||||
| SuperDrug CAS ID |
cas=000768945
|
||||
| Drug Resistance Mutation (DRM) | |||||
| DRM | DRM Info | ||||
| Target and Pathway | |||||
| Target(s) | Influenza A virus M2 protein | Target Info | Inhibitor | [535075], [535790], [536654], [537379] | |
| WikiPathways | Influenza Life Cycle | ||||
| References | |||||
| Ref 467472 | (http://www.guidetopharmacology.org/) Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 4128). | ||||
| Ref 537115 | Emerging treatments for traumatic brain injury. Expert Opin Emerg Drugs. 2009 Mar;14(1):67-84. | ||||
| Ref 535075 | pH-dependent tetramerization and amantadine binding of the transmembrane helix of M2 from the influenza A virus. Biochemistry. 2000 Nov 21;39(46):14160-70. | ||||
| Ref 535790 | Proton conduction through the M2 protein of the influenza A virus; a quantitative, mechanistic analysis of experimental data. FEBS Lett. 2003 Sep 18;552(1):17-22. | ||||
| Ref 536654 | Current and future antiviral therapy of severe seasonal and avian influenza. Antiviral Res. 2008 Apr;78(1):91-102. Epub 2008 Feb 4. | ||||
| Ref 537379 | Discovery of spiro-piperidine inhibitors and their modulation of the dynamics of the M2 proton channel from influenza A virus. J Am Chem Soc. 2009 Jun 17;131(23):8066-76. | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

