Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D01TMQ
|
||||
| Former ID |
DCL000733
|
||||
| Drug Name |
Bupropion+naltrexone
|
||||
| Synonyms |
Contrave (TN)
|
||||
| Drug Type |
Small molecular drug
|
||||
| Indication | Obesity [ICD9: 278; ICD10:E66] | Phase 3 | [1] | ||
| Company |
Orexigen Therapeutics
|
||||
| Structure |
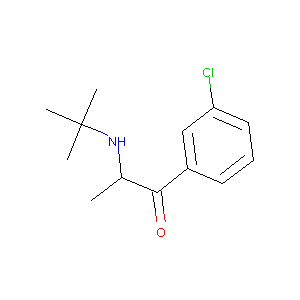
|
Download2D MOL |
|||
| Formula |
C13H18ClNO
|
||||
| PubChem Compound ID | |||||
| Target and Pathway | |||||
| Target(s) | Dopamine reuptake | Target Info | Inhibitor | [2] | |
| Sodium-dependent noradrenaline transporter | Target Info | Inhibitor | [2] | ||
| Opioid receptor | Target Info | Antagonist | [2] | ||
| PANTHER Pathway | Adrenaline and noradrenaline biosynthesis | ||||
| Reactome | Na+/Cl- dependent neurotransmitter transporters | ||||
| WikiPathways | Monoamine Transport | ||||
| NRF2 pathway | |||||
| Transport of glucose and other sugars, bile salts and organic acids, metal ions and amine compounds | |||||
| References | |||||
| REF 1 | A randomized, phase 3 trial of naltrexone SR/bupropion SR on weight and obesity-related risk factors (COR-II). Obesity (Silver Spring). 2013 May;21(5):935-43. | ||||
| REF 2 | Anti-obesity drugs. Expert Opin Pharmacother. 2008 Jun;9(8):1339-50. | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

