Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D01LYX
|
||||
| Former ID |
DIB009619
|
||||
| Drug Name |
Eptastigmine
|
||||
| Synonyms |
Epiastigmine; Eptastigmine tartrate; Heptylstigmine tartrate; Heptyl-physostigmine; L-693487; MF-201
|
||||
| Drug Type |
Small molecular drug
|
||||
| Indication | Cognitive disorders [ICD9: 290-294, 294.0, 780.09, 780.9, 780.93; ICD10:F01-F07, F04, F05, R41.3] | Phase 3 | [1] | ||
| Company |
Consiglio Nazionale delle Ricerche
|
||||
| Structure |
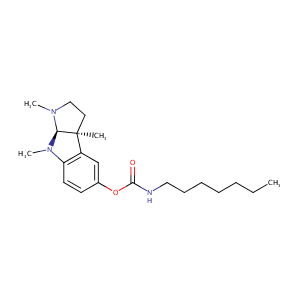
|
Download2D MOL |
|||
| Formula |
C21H33N3O2
|
||||
| Canonical SMILES |
[C@]12([C@@H](N(c3c1cc(OC(=O)NCCCCCCC)cc3)C)N(CC2)C)C
|
||||
| PubChem Compound ID | |||||
| Target and Pathway | |||||
| Target(s) | Acetylcholinesterase | Target Info | Inhibitor | [1] | |
| KEGG Pathway | Glycerophospholipid metabolism | ||||
| Cholinergic synapse | |||||
| PANTHER Pathway | Muscarinic acetylcholine receptor 1 and 3 signaling pathway | ||||
| Muscarinic acetylcholine receptor 2 and 4 signaling pathway | |||||
| Nicotinic acetylcholine receptor signaling pathway | |||||
| Pathway Interaction Database | ATF-2 transcription factor network | ||||
| PathWhiz Pathway | Phospholipid Biosynthesis | ||||
| WikiPathways | Monoamine Transport | ||||
| Biogenic Amine Synthesis | |||||
| Acetylcholine Synthesis | |||||
| Integrated Pancreatic Cancer Pathway | |||||
| References | |||||
| REF 1 | Eptastigmine: ten years of pharmacology, toxicology, pharmacokinetic, and clinical studies. CNS Drug Rev. 2001 Winter;7(4):369-86. | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

