Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D01HKZ
|
||||
| Former ID |
DIB004927
|
||||
| Drug Name |
TSR-011
|
||||
| Indication | Non-small cell lung cancer [ICD10:C33-C34] | Phase 1/2 | [1] | ||
| Company |
Tesaro
|
||||
| Structure |
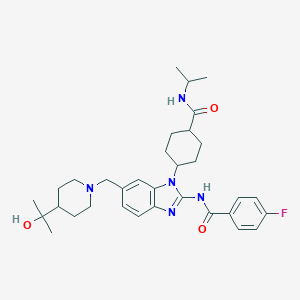
|
Download2D MOL |
|||
| Target and Pathway | |||||
| Target(s) | ALK tyrosine kinase receptor | Target Info | Inhibitor | [2], [3] | |
| KEGG Pathway | Non-small cell lung cancer | ||||
| WikiPathways | Differentiation Pathway | ||||
| References | |||||
| REF 1 | ClinicalTrials.gov (NCT02048488) A Phase I/IIa Open-Label, Dose Escalation and Cohort Expansion Trial of Oral TSR-011 in Patients With Advanced Solid Tumors and Lymphomas. U.S. National Institutes ofHealth. | ||||
| REF 2 | Treatment of ALK-positive non-small cell lung cancer. Arch Pathol Lab Med. 2012 Oct;136(10):1201-4. | ||||
| REF 3 | National Cancer Institute Drug Dictionary (drug id 757983). | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

