| Drug General Information |
| Drug ID |
D00QSV
|
| Former ID |
DNC012388
|
| Drug Name |
2-(3-Methoxy-phenyl)-1-methyl-ethylamine
|
| Drug Type |
Small molecular drug
|
| Indication |
Discovery agent
|
Investigative |
[1]
|
|---|
| Structure |
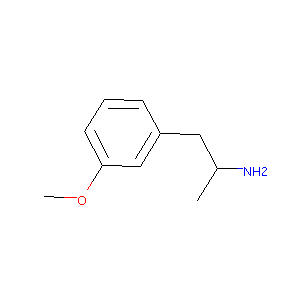
|
Download
2D MOL
3D MOL
|
| Formula |
C10H15NO
|
| Canonical SMILES |
CC(CC1=CC(=CC=C1)OC)N
|
| InChI |
1S/C10H15NO/c1-8(11)6-9-4-3-5-10(7-9)12-2/h3-5,7-8H,6,11H2,1-2H3
|
| InChIKey |
VEJWNIYARKAHFI-UHFFFAOYSA-N
|
| PubChem Compound ID |
|
| Target and Pathway |
| Target(s) |
5-hydroxytryptamine 2A receptor |
Target Info |
Inhibitor |
[1]
|
|---|
| 5-hydroxytryptamine 1A receptor |
Target Info |
Inhibitor |
[1]
|
| 5-hydroxytryptamine 1D receptor |
Target Info |
Inhibitor |
[1]
|
|
KEGG Pathway
|
Calcium signaling pathway
|
|
Neuroactive ligand-receptor interaction
|
|
Gap junction
|
|
Serotonergic synapse
|
|
Inflammatory mediator regulation of TRP channelshsa04024:cAMP signaling pathway
|
|
Serotonergic synapsehsa04024:cAMP signaling pathway
|
|
PANTHER Pathway
|
5HT2 type receptor mediated signaling pathwayP00026:Heterotrimeric G-protein signaling pathway-Gi alpha and Gs alpha mediated pathway
|
|
5HT1 type receptor mediated signaling pathwayP00026:Heterotrimeric G-protein signaling pathway-Gi alpha and Gs alpha mediated pathway
|
|
5HT1 type receptor mediated signaling pathway
|
|
Reactome
|
Serotonin receptors
|
|
G alpha (q) signalling eventsR-HSA-390666:Serotonin receptors
|
|
G alpha (i) signalling eventsR-HSA-390666:Serotonin receptors
|
|
G alpha (i) signalling events
|
|
WikiPathways
|
Serotonin Receptor 2 and STAT3 Signaling
|
|
Serotonin Receptor 2 and ELK-SRF/GATA4 signaling
|
|
SIDS Susceptibility Pathways
|
|
Monoamine GPCRs
|
|
GPCRs, Class A Rhodopsin-like
|
|
Gastrin-CREB signalling pathway via PKC and MAPK
|
|
GPCR ligand binding
|
|
GPCR downstream signaling
|
|
GPCRs, OtherWP722:Serotonin HTR1 Group and FOS Pathway
|
|
GPCR downstream signalingWP722:Serotonin HTR1 Group and FOS Pathway
|
| References |
| REF 1 | J Med Chem. 1986 Feb;29(2):194-9.5-HT1 and 5-HT2 binding characteristics of 1-(2,5-dimethoxy-4-bromophenyl)-2-aminopropane analogues. |
|---|