Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D00HBE
|
||||
| Former ID |
DIB014053
|
||||
| Drug Name |
AZD0914
|
||||
| Indication | Bacterial infections [ICD9: 001-009, 010-018, 020-027, 030-041, 080-088, 090-099, 100-104; ICD10:A00-B99] | Phase 2 | [524944] | ||
| Company |
Astrazeneca
|
||||
| Structure |
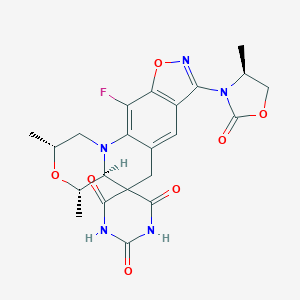
|
Download2D MOL |
|||
| Target and Pathway | |||||
| Target(s) | Bacterial DNA gyrase | Target Info | Modulator | ||
| References | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

