Target expression details
| Target General Information | |||||
|---|---|---|---|---|---|
| Target ID | T40016 | ||||
| Target Name | Glucocorticoid receptor | ||||
| Synonyms | Alpha-A; GR; NR3C1 | ||||
| Target Type | Successful | ||||
| Gene Name | NR3C1 | ||||
| Biochemical Class | Zinc-finger | ||||
| UniProt ID | GCR_HUMAN | ||||
| Target Gene Expression Profiles in the Disease-Relevant Drug Targeted Tissue of the Patients and Healthy Individuals | |||||
| Disease | Asthma | ||||
| Example drug | Fluticasone furoate | Approved | [522134], [551873], [1572592] | ||
| Tissue | Nasal and bronchial airway | ||||
| Level of differential expression between the patients in the disease section of the tissue and the tissues of healthy individual | Fold-change: 4.82E-03 Z-score: 6.42E-03 P-value: 5.51E-01 |
||||
|
Target gene expression profiles of the patients in the disease section of the tissue
Target gene expression profiles in the tissue of healthy individual

|
|||||
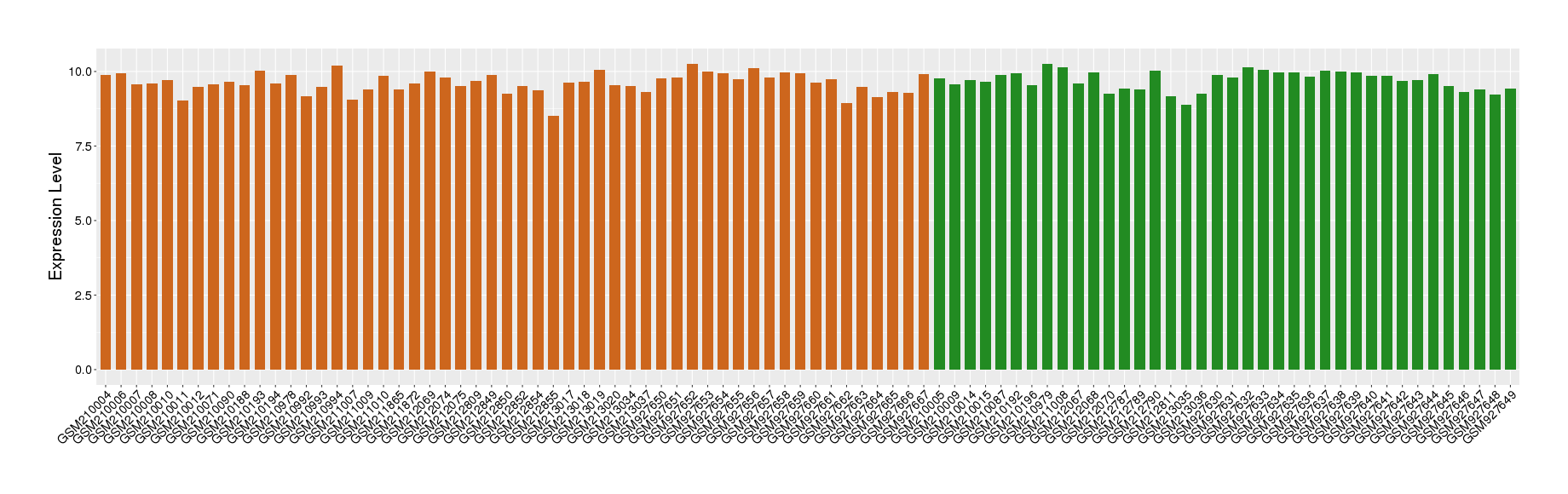
| Disease | Chronic obstructive pulmonary disease | ||||
| Example drug | Fluticasone furoate | Approved | [522134], [551873], [1572592] | ||
| Tissue | Lung tissue | ||||
| Level of differential expression between the patients in the disease section of the tissue and the tissues of healthy individual | Fold-change: -0.16 Z-score: -0.49 P-value: 2.10E-01 |
||||
|
Target gene expression profiles of the patients in the disease section of the tissue
Target gene expression profiles in the tissue of healthy individual
|
|||||
| Disease | Chronic obstructive pulmonary disease | ||||
| Example drug | Fluticasone furoate | Approved | [522134], [551873], [1572592] | ||
| Tissue | Small airway epithelium | ||||
| Level of differential expression between the patients in the disease section of the tissue and the tissues of healthy individual | Fold-change: -0.12 Z-score: -0.32 P-value: 5.12E-03 |
||||
|
Target gene expression profiles of the patients in the disease section of the tissue
Target gene expression profiles in the tissue of healthy individual

|
|||||
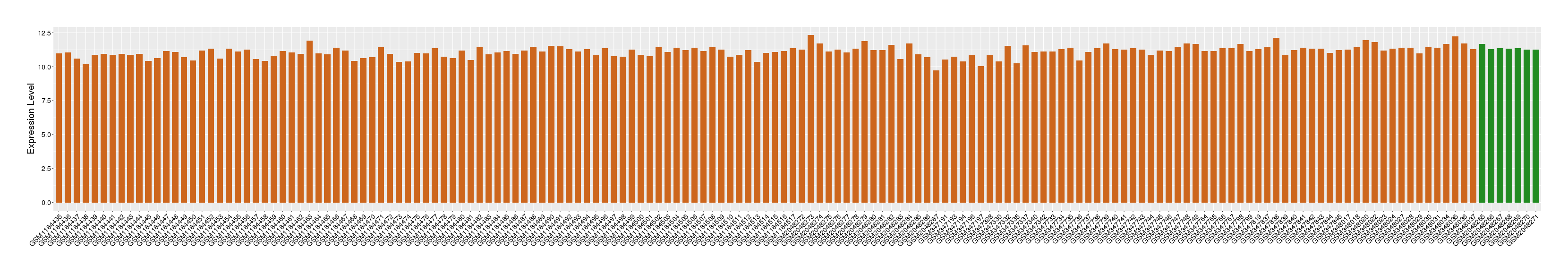
| Disease | Rheumatoid arthritis | ||||
| Example drug | Dexamethasone | Approved | [536737], [540387], [1572592] | ||
| Tissue | Synovial tissue | ||||
| Level of differential expression between the patients in the disease section of the tissue and the tissues of healthy individual | Fold-change: -0.15 Z-score: -1.01 P-value: 3.40E-03 |
||||
|
Target gene expression profiles of the patients in the disease section of the tissue
Target gene expression profiles in the tissue of healthy individual
|
|||||
| Disease | Multiple sclerosis | ||||
| Example drug | Prednisolone | Phase 3 | [551871], [1572592] | ||
| Tissue | Spinal cord | ||||
| Level of differential expression between the patients in the disease section of the tissue and the patients in the normal section of the tissue adjacent to the disease section | Fold-change: 0.13 Z-score: 0.20 P-value: 5.81E-01 |
||||
|
Target gene expression profiles of the patients in the disease section of the tissue
Target gene expression profiles of the patients in the normal section of the tissue adjacent to the disease section

|
|||||
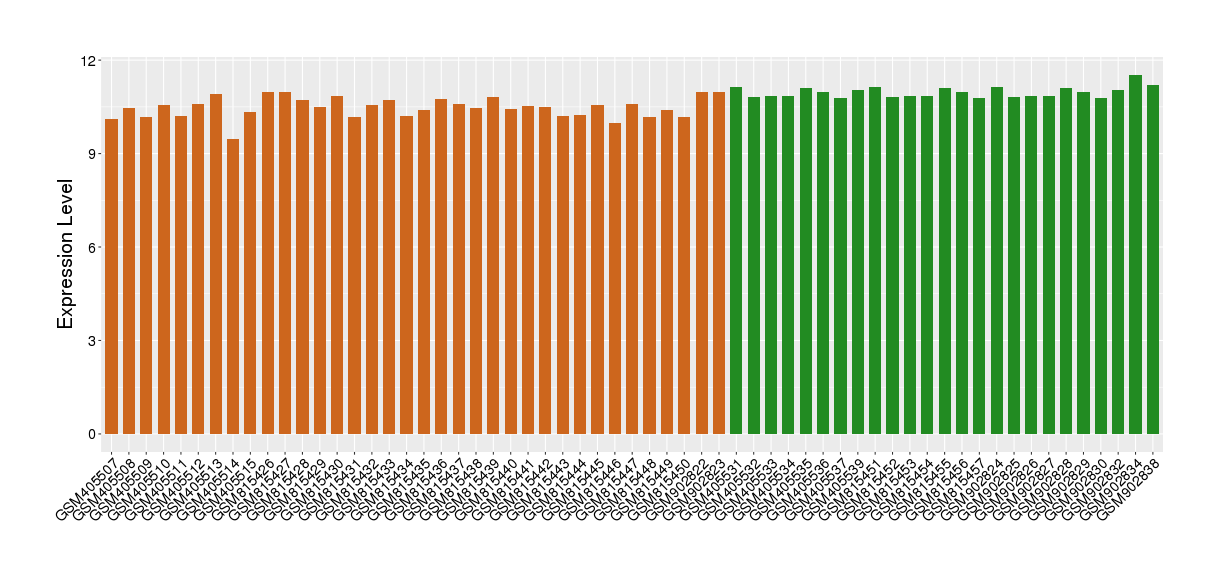
| Disease | Atopic dermatitis | ||||
| Example drug | GSK870086 | Phase 2 | [522149], [523364], [1572592] | ||
| Tissue | Skin | ||||
| Level of differential expression between the patients in the disease section of the tissue and the tissues of healthy individual | Fold-change: -0.49 Z-score: -2.70 P-value: 3.81E-10 |
||||
|
Target gene expression profiles of the patients in the disease section of the tissue
Target gene expression profiles in the tissue of healthy individual
|
|||||
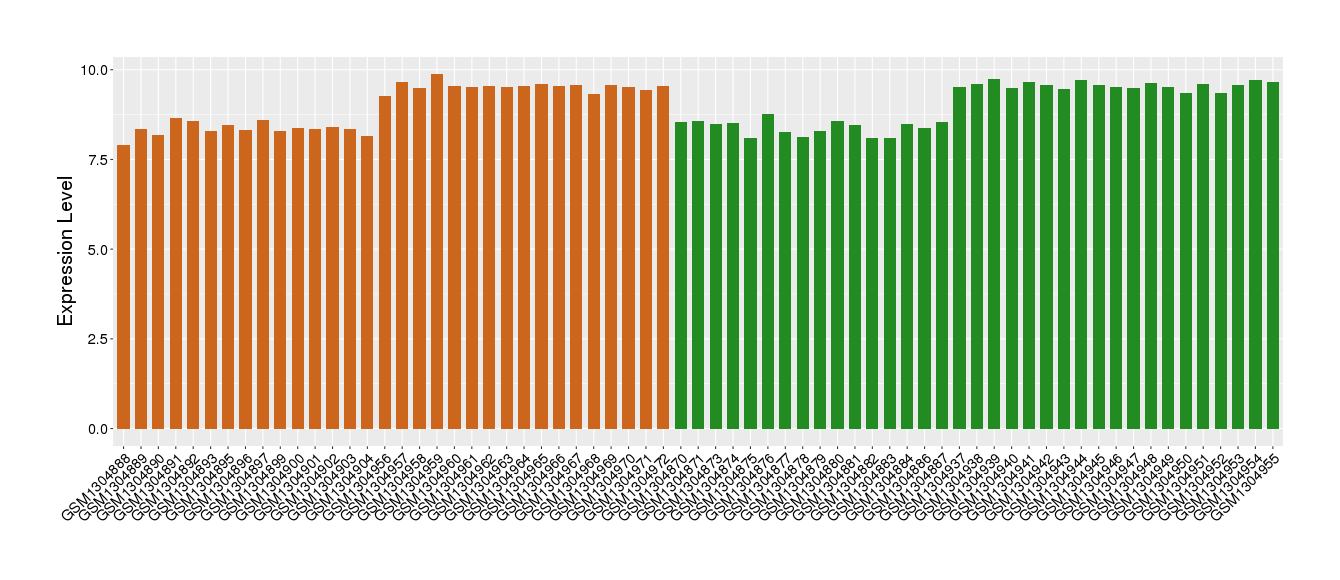
| Disease | Depression | ||||
| Example drug | ORG-34517 | Phase 2 | [521717], [1572592] | ||
| Tissue | Pre-frontal cortex | ||||
| Level of differential expression between the patients in the disease section of the tissue and the tissues of healthy individual | Fold-change: -0.04 Z-score: -0.07 P-value: 7.57E-01 |
||||
|
Target gene expression profiles of the patients in the disease section of the tissue
Target gene expression profiles in the tissue of healthy individual
|
|||||
| Target Gene Expression Profiles in Other Tissues of Healthy Individuals | |||||
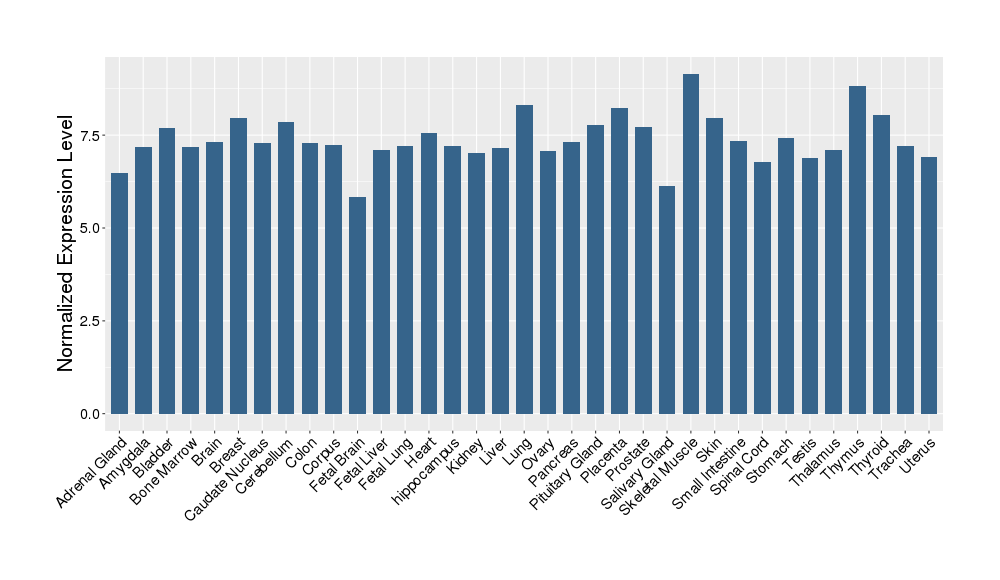
|
|||||
| Reference | |||||
| Ref 540387 | (http://www.guidetopharmacology.org/) Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 3447). | ||||
| Ref 536737 | Emerging drugs for idiopathic thrombocytopenic purpura in adults. Expert Opin Emerg Drugs. 2008 Jun;13(2):237-54. | ||||
| Ref 521717 | ClinicalTrials.gov (NCT00212797) A Study to Determine the Efficacy and Safety of 2 Doses of Org 34517 as Adjunctive Therapy in Subjects With Psychotic Major Depression (28130)(P05845). U.S. National Institutes of Health. | ||||
| Ref 522134 | ClinicalTrials.gov (NCT00539006) Comparator Study Evaluating Patient Preference Of FFNS vs. FPNS. U.S. National Institutes of Health. | ||||
| Ref 522149 | ClinicalTrials.gov (NCT00549497) A Randomized Study Evaluating Steroid Hormone Levels, Safety And Tolerability Of GW870086X In Healthy Volunteers. U.S. National Institutes of Health. | ||||
| Ref 523364 | ClinicalTrials.gov (NCT01299610) A Study to Test the Effect of 2 Different Doses of Topical GW870086X on Atopic Dermatitis Also Including a Postive Control and a Placebo. U.S. National Institutes of Health. | ||||
| Ref 551871 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015 | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

