Target expression details
| Target General Information | |||||
|---|---|---|---|---|---|
| Target ID | T20761 | ||||
| Target Name | Vascular endothelial growth factor A | ||||
| Synonyms | VEGF-A; Vascular permeability factor; VPF; VEGFA | ||||
| Target Type | Successful | ||||
| Gene Name | VEGFA | ||||
| Biochemical Class | PDGF VEGF growth factor | ||||
| UniProt ID | VEGFA_HUMAN | ||||
| Target Gene Expression Profiles in the Disease-Relevant Drug Targeted Tissue of the Patients and Healthy Individuals | |||||
| Disease | Rectal cancer | ||||
| Example drug | Aflibercept | Approved | [551871], [1572592] | ||
| Tissue | Rectal colon tissue | ||||
| Level of differential expression between the patients in the disease section of the tissue and the tissues of healthy individual | Fold-change: 1.20 Z-score: 2.26 P-value: 2.07E-03 |
||||
| Level of differential expression between the patients in the disease section of the tissue and the patients in the normal section of the tissue adjacent to the disease section | Fold-change: 0.97 Z-score: 4.06 P-value: 1.28E-05 |
||||
|
Target gene expression profiles of the patients in the disease section of the tissue
Target gene expression profiles of the patients in the normal section of the tissue adjacent to the disease section
Target gene expression profiles in the tissue of healthy individual
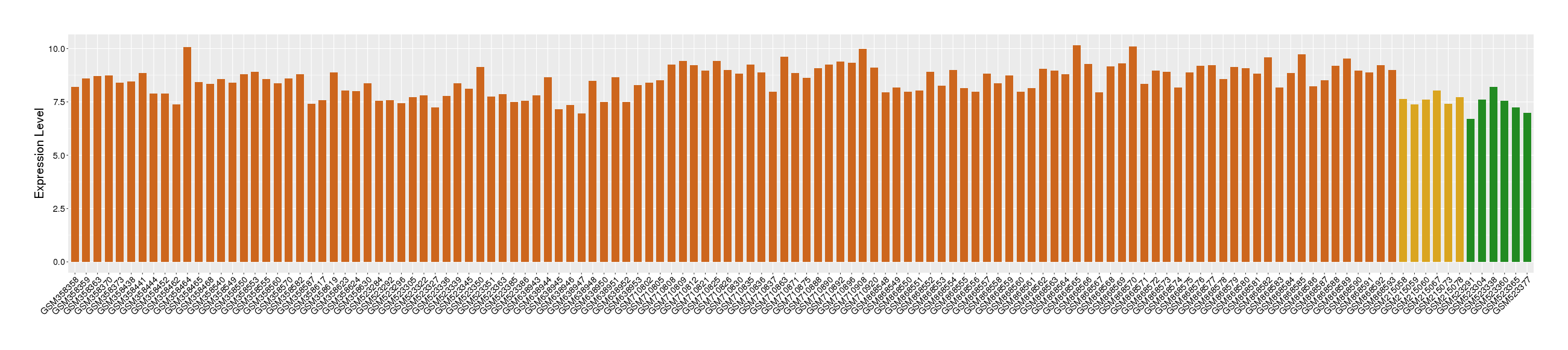
|
|||||
| Disease | Lateral sclerosis | ||||
| Example drug | SNN-0029 | Phase 1/2 | [523531], [1572592] | ||
| Tissue | Cervical spinal cord | ||||
| Level of differential expression between the patients in the disease section of the tissue and the tissues of healthy individual | Fold-change: 0.15 Z-score: 0.47 P-value: 3.61E-01 |
||||
|
Target gene expression profiles of the patients in the disease section of the tissue
Target gene expression profiles in the tissue of healthy individual

|
|||||
| Target Gene Expression Profiles in Other Tissues of Healthy Individuals | |||||
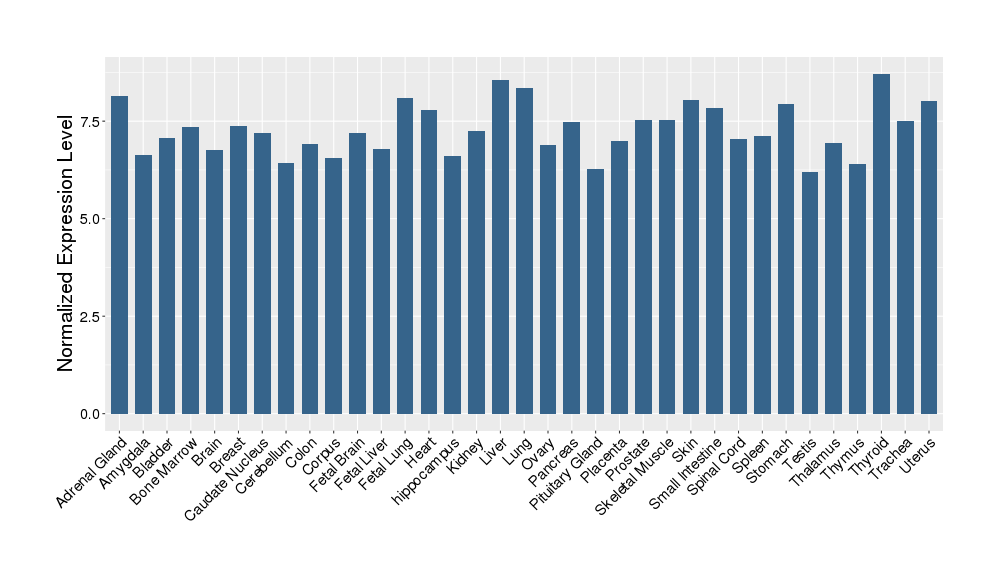
|
|||||
| Reference | |||||
| Ref 523531 | ClinicalTrials.gov (NCT01384162) An Open Label, Safety and Tolerability Continuation Study of Intracerebroventricular Administration of sNN0029 to Patients With Amyotrophic Lateral Sclerosis. U.S. National Institutes of Health. | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

