Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D06LRG
|
||||
| Former ID |
DIB001593
|
||||
| Drug Name |
TRV027
|
||||
| Indication | Heart failure [ICD9: 428; ICD10:I50] | Phase 2 | [524481] | ||
| Company |
Forest laboratories; trevena
|
||||
| Structure |
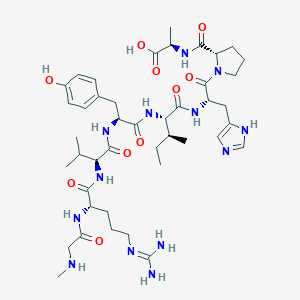
|
Download2D MOL |
|||
| Target and Pathway | |||||
| Target(s) | Type-1 angiotensin II receptor | Target Info | Antagonist | [532415] | |
| NetPath Pathway | TGF_beta_Receptor Signaling Pathway | ||||
| Pathway Interaction Database | Arf6 trafficking events | ||||
| Arf6 signaling events | |||||
| Angiopoietin receptor Tie2-mediated signaling | |||||
| PathWhiz Pathway | Angiotensin Metabolism | ||||
| Muscle/Heart Contraction | |||||
| References | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

