Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D0O0JP
|
||||
| Former ID |
DIB000730
|
||||
| Drug Name |
EMA-401
|
||||
| Synonyms |
Neuropathic pain targeting small molecules (postherpetic neuralgia), Spinifex
|
||||
| Drug Type |
Small molecular drug
|
||||
| Company |
Spinifex pharmaceuticals
|
||||
| Structure |
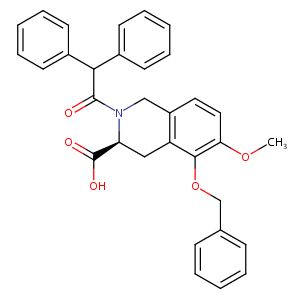
|
Download2D MOL |
|||
| Formula |
C32H29NO5
|
||||
| InChI |
InChI=1S/C32H29NO5/c1-37-28-18-17-25-20-33(31(34)29(23-13-7-3-8-14-23)24-15-9-4-10-16-24)27(32(35)36)19-26(25)30(28)38-21-22-11-5-2-6-12-22/h2-18,27,29H,19-21H2,1H3,(H,35,36)/t27-/m0/s1
|
||||
| InChIKey |
GHBCIXGRCZIPNQ-MHZLTWQESA-N
|
||||
| PubChem Compound ID | |||||
| PubChem Substance ID | |||||
| Target and Pathway | |||||
| Target(s) | Type-2 angiotensin II receptor | Target Info | Antagonist | [551699] | |
| References | |||||
| Ref 525181 | ClinicalTrials.gov (NCT02435199) Clinical Trial Designed to Determine the Safety and Efficacy of EMA401 in Patients With Painful Diabetic Neuropathy. U.S. National Institutes of Health. | ||||
| Ref 543095 | (http://www.guidetopharmacology.org/) Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 8374). | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

