Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D0U5OU
|
||||
| Former ID |
DIB011521
|
||||
| Drug Name |
Naproxcinod
|
||||
| Synonyms |
Beprana; Nitronaproxen; AR-P900758XX; AZD-3582; HCT-3012; Nitronaproxen, NicOx; NO-naproxen, AstraZeneca; NO-naproxen, NicOx; NO-NSAID, NicOx/AstraZeneca; Nitric oxide-donating derivative of naproxen (oral, osteoarthritis), NicOx
|
||||
| Drug Type |
Small molecular drug
|
||||
| Indication | Inflammatory disease [ICD9: 140-229, 147, 173, 573.3, 710-719; ICD10:C11, C44, K75.9, M00-M25] | Phase 3 | [522137] | ||
| Company |
NicOx SA
|
||||
| Structure |
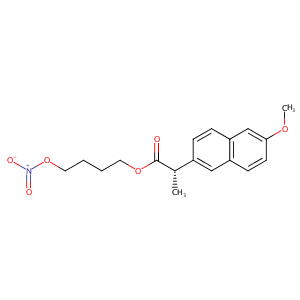
|
Download2D MOL |
|||
| Formula |
C18H21NO6
|
||||
| Canonical SMILES |
[N+](=O)([O-])OCCCCOC(=O)[C@H](c1cc2c(cc(cc2)OC)cc1)C
|
||||
| CAS Number |
CAS 163133-43-5
|
||||
| PubChem Compound ID | |||||
| Target and Pathway | |||||
| Target(s) | Cyclooxygenase | Target Info | Modulator | [537263] | |
| References | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

