Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D01SHZ
|
||||
| Former ID |
DNCL002606
|
||||
| Drug Name |
Rucaparib
|
||||
| Drug Type |
Small molecular drug
|
||||
| Company |
Clovis Oncology
|
||||
| Structure |
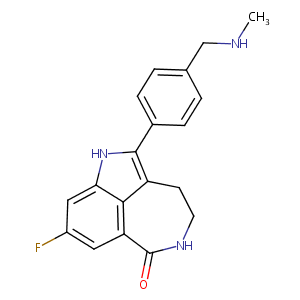
|
Download2D MOL |
|||
| Formula |
C19H18FN3O
|
||||
| InChI |
InChI=1S/C19H18FN3O/c1-21-10-11-2-4-12(5-3-11)18-14-6-7-22-19(24)15-8-13(20)9-16(23-18)17(14)15/h2-5,8-9,21,23H,6-7,10H2,1H3,(H,22,24)
|
||||
| InChIKey |
HMABYWSNWIZPAG-UHFFFAOYSA-N
|
||||
| PubChem Compound ID | |||||
| PubChem Substance ID |
16463842, 24207084, 76934109, 103769588, 103905261, 121278129, 128080164, 135626799, 137263927, 137276017, 142270192, 152037846, 162108736, 162829534, 164208495, 170474818, 172918029, 174006354, 177748908, 185992962, 194944026, 198980201, 210274713, 210280347, 223366069, 223471430, 223804825, 227116711, 249625884
|
||||
| Target and Pathway | |||||
| Target(s) | Poly ADP ribose polymerase (PARP) | Target Info | Modulator | [889440] | |
| References | |||||
| Ref 524484 | ClinicalTrials.gov (NCT01968213) A Study of Rucaparib as Switch Maintenance Following Platinum-Based Chemotherapy in Patients With Platinum-Sensitive, High-Grade Serous or Endometrioid Epithelial Ovarian, Primary Peritoneal or Fallopian Tube Cancer. U.S. National Institutes of Health. | ||||
| Ref 542704 | (http://www.guidetopharmacology.org/) Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7736). | ||||
| Ref 889440 | 2016 FDA drug approvals. Nat Rev Drug Discov. 2017 Feb 2;16(2):73-76. doi: 10.1038/nrd.2017.14. | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

