Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D03IUD
|
||||
| Former ID |
DIB013340
|
||||
| Drug Name |
Ceftolozane sulfate
|
||||
| Synonyms |
Ceftolozane; CXA-101; CXA-301; CXA-301); Cephalosporin derivatives, Astellas; Cephalosporinderivatives, Calixa Therapeutics; FR-193879; FR-264205; FR-295389; CXA-101 (inhaled), Calixa; CXA-101 (inhaled), Cubist; Cephalosporin derivative (H pylori/P aeruginosa infection), Astellas; CXA-101 (inhaled, bacterial lung infection), Cubist
|
||||
| Drug Type |
Small molecular drug
|
||||
| Indication | Bacterial respiratory tract infection [ICD10:J13, J15] | Phase 4 | [525160] | ||
| Company |
Fujisawa Pharmaceutical Co Ltd; Calixa Therapeutics Inc
|
||||
| Structure |
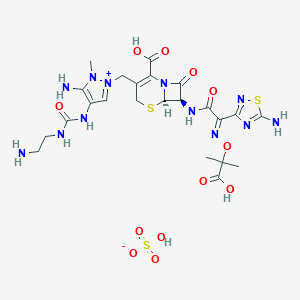
|
Download2D MOL |
|||
| Formula |
C23H32N12O12S3
|
||||
| Canonical SMILES |
CC(C)(O/N=C(\c1nc(sn1)N)/C(=O)N[C@H]1[C@H]2SCC(=C(N2C1=<br />O)C(=O)O)C[n+]1cc(c(n1C)N)NC(=O)NCCN)C(=O)O.S(=O)(=O)(O<br />)[O-]
|
||||
| PubChem Compound ID | |||||
| Target and Pathway | |||||
| Target(s) | Bacterial penicillin binding protein | Target Info | Modulator | [1572591] | |
| References | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

