Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D0D7UD
|
||||
| Former ID |
DIB002523
|
||||
| Drug Name |
AS-013
|
||||
| Synonyms |
Circulase; Ecraprost; Prostaglandin E1 (liposomal), Mitsubishi Pharma/Asahi Glass
|
||||
| Drug Type |
Small molecular drug
|
||||
| Indication | Thrombosis [ICD9: 437.6, 453, 671.5, 671.9; ICD10:I80-I82] | Phase 3 | [534768] | ||
| Company |
Taisho Pharmaceutical Co Ltd
|
||||
| Structure |
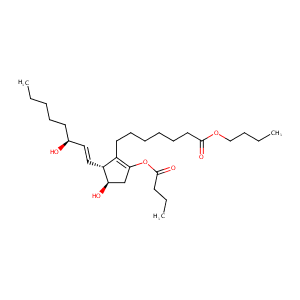
|
Download2D MOL |
|||
| Formula |
C28H48O6
|
||||
| Canonical SMILES |
C1(=C([C@@H](/C=C/[C@@H](O)CCCCC)[C@@H](C1)O)CCCCCCC(=O<br />)OCCCC)OC(=O)CCC
|
||||
| CAS Number |
CAS 136892-64-3
|
||||
| PubChem Compound ID | |||||
| Target and Pathway | |||||
| Target(s) | Prostaglandin E2 receptor, EP1 subtype | Target Info | Agonist | [534768] | |
| PANTHER Pathway | PI3 kinase pathway | ||||
| References | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

