Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D0X1BJ
|
||||
| Former ID |
DIB001326
|
||||
| Drug Name |
Denibulin
|
||||
| Synonyms |
Denibulin hydrochloride; ANG-600 series; ANG-615; MN-029; Second generation VTAs, Angiogene Pharmaceuticals; Second generation VTAs, MediciNova; Vascular targeting agents (benzimidazole carbamates), Angiogene Pharmaceuticals; Vascular targeting agents (benzimidazole carbamates), MediciNova
|
||||
| Drug Type |
Small molecular drug
|
||||
| Indication | Cancer [ICD9: 140-229; ICD10:C00-C96] | Discontinued in Phase 1 | [548126] | ||
| Company |
Angiogene Pharmaceuticals Ltd
|
||||
| Structure |
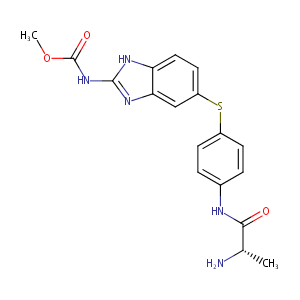
|
Download2D MOL |
|||
| Formula |
C18H19N5O3S
|
||||
| Canonical SMILES |
c12c([nH]c(n1)NC(=O)OC)ccc(c2)Sc1ccc(cc1)NC(=O)[C@H](C)<br />N
|
||||
| PubChem Compound ID | |||||
| Target and Pathway | |||||
| Target(s) | Tubulin beta | Target Info | Inhibitor | [544311] | |
| PANTHER Pathway | Cytoskeletal regulation by Rho GTPase | ||||
| Huntington disease | |||||
| References | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

