Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D04RLY
|
||||
| Former ID |
DAP000949
|
||||
| Drug Name |
Vindesine
|
||||
| Synonyms |
DAVA; Vindesin; Vindesina; Vindesinum; Desacetylvinblastine amide; Lilly 112531; Vindesina [INN-Spanish]; Vindesinum [INN-Latin]; Vindesine (USAN/INN); Vindesine [USAN:BAN:INN]; Vindesine [USAN:INN:BAN]; 3-(Aminocarbonyl)-O(sup 4)-deacetyl-3-de(methoxycarbonyl)vincaleukoblastine; 3-(aminocarbonyl)-O(4)-deacetyl-3-de(methoxycarbonyl)vincaleukoblastine; 3-Carbamoyl-4-deacetyl-3-de(methoxycarbonyl)vincaleukoblastine; 3-carbamoyl-O(4)-deacetyl-3-de(methoxycarbonyl)vincaleukoblastine; Methyl (5S,7S,9S)-9-[(2b,3b,4b,5a,12b,19a)-3-carbamoyl-3,4-dihydroxy-16-methoxy-1-methyl-6,7-didehydroaspidospermidin-15-yl]-5-ethyl-5-hydroxy-1,4,5,6,7,8,9,10-octahydro-2H-3,7-methanoazacycloundecino[5,4-b]indole-9-carboxylate; Methyl (5S,7S,9S)-9-[(2beta,3beta,4beta,5alpha,12beta,19alpha)-3-carbamoyl-3,4-dihydroxy-16-methoxy-1-methyl-6,7-didehydroaspidospermidin-15-yl]-5-ethyl-5-hydroxy-1,4,5,6,7,8,9,10-octahydro-2H-3,7-methanoazacycloundecino[5,4-b]indole-9-carboxylate
|
||||
| Drug Type |
Small molecular drug
|
||||
| Therapeutic Class |
Anticancer Agents
|
||||
| Structure |
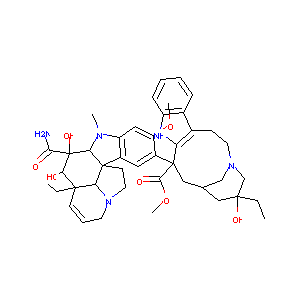
|
Download2D MOL |
|||
| Formula |
C43H55N5O7
|
||||
| Canonical SMILES |
CCC1(CC2CC(C3=C(CCN(C2)C1)C4=CC=CC=C4N3)(C5=C(C=C6C(=C5<br />)C78CCN9C7C(C=CC9)(C(C(C8N6C)(C(=O)N)O)O)CC)OC)C(=O)OC)<br />O
|
||||
| InChI |
1S/C43H55N5O7/c1-6-39(52)21-25-22-42(38(51)55-5,33-27(13-17-47(23-25)24-39)26-11-8-9-12-30(26)45-33)29-19-28-31(20-32(29)54-4)46(3)35-41(28)15-18-48-16-10-14-40(7-2,34(41)48)36(49)43(35,53)37(44)50/h8-12,14,19-20,25,34-36,45,49,52-53H,6-7,13,15-18,21-24H2,1-5H3,(H2,44,50)/t25-,34+,35-,36-,39+,40-,41-,42+,43+/m1/s1
|
||||
| InChIKey |
HHJUWIANJFBDHT-KOTLKJBCSA-N
|
||||
| CAS Number |
CAS 59917-39-4
|
||||
| PubChem Compound ID | |||||
| PubChem Substance ID |
7980884, 8176819, 14937951, 24712319, 34706568, 46504548, 47207961, 48416698, 50063064, 53788169, 57312475, 77478141, 92309091, 102853537, 103504328, 104335455, 126686646, 127301247, 127301248, 127301249, 127301250, 127301251, 127301252, 127301253, 127301254, 127301255, 127301256, 127301257, 127301258, 127301259, 127301260, 127301261, 127301262, 127301263, 127301264, 127301265, 127301266, 127301267, 127301268, 127301269, 127301270, 127301271, 127301272, 127301273, 127301274, 127301275, 127301276, 127301277, 127301278, 134338223
|
||||
| ChEBI ID |
ChEBI:32295
|
||||
| SuperDrug ATC ID |
L01CA03
|
||||
| SuperDrug CAS ID |
cas=053643484
|
||||
| Target and Pathway | |||||
| Target(s) | Tubulin beta | Target Info | Binder | [536411], [536504] | |
| PANTHER Pathway | Cytoskeletal regulation by Rho GTPase | ||||
| Huntington disease | |||||
| References | |||||
| Ref 536411 | Antiproliferating activity of the mitotic inhibitor pironetin against vindesine- and paclitaxel-resistant human small cell lung cancer H69 cells. Anticancer Res. 2007 Mar-Apr;27(2):729-36. | ||||
| Ref 536504 | Gene signatures developed from patient tumor explants grown in nude mice to predict tumor response to 11 cytotoxic drugs. Cancer Genomics Proteomics. 2007 May-Jun;4(3):197-209. | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

