Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D06OIV
|
||||
| Former ID |
DAP000136
|
||||
| Drug Name |
Tolbutamide
|
||||
| Synonyms |
Aglicid; Arcosal; Arkozal; Artosin; Artozin; Beglucin; Butamid; Butamide; Butamidum; Diaben; Diabesan; Diabetamid; Diabetol; Diabuton; Diasulfon; Diaval; Dirastan; Dolipol; Drabet; Glyconon; Ipoglicone; Mobenol; Orabet; Oralin; Oramide; Orezan; Orinase; Orinaz; Oterben; Pramidex; Rastinon; Restinon; Tarasina; Tolbet;Tolbusal; Tolbutamid; Tolbutamida; Tolbutamidum; Tolbutone; Toluina; Tolumid; Toluran; Toluvan; Tolylsulfonylbutylurea; Willbutamide; Apotex Brand of Tolbutamide; Aventis Brand of Tolbutamide; BerlinChemie Brand of Tolbutamide; Butamide Brand of Tolbutamide; Hoechst Brand of Tolbutamide; Pfizer Brand of Tolbutamide; TOLBUTAMIDE USP; Tolbutamide Aventis Brand; Tolbutamide Butamide Brand; Tolbutamide Hoechst Brand; Tolbutamide Pfizer Brand; Valdecasas Brand of Tolbutamide; Yamanouchi Brand of Tolbutamide; D 860; HLS 831; T 0891; U 2043; Apo-Tolbutamide; Berlin-Chemie Brand of Tolbutamide; Novo-Butamide; Orinase (TN); Sk-tolbutamide; Tol-Tab; Tolbutamida [INN-Spanish]; Tolbutamidum [INN-Latin]; Tolbutamide [INN:BAN:JAN]; R.A.N. Brand of Tolbutamide; Tolbutamid R.A.N.; Tolbutamide (JP15/USP/INN); N-4-Methylbenzolsulfonyl-N-butylurea; N-4-(Methylbenzolsulfonyl)-n-butylurea; N-Butyl-N'-p-toluenesulfonylurea; N-n-Butyl-N'-tosylurea; N-(4-Methylbenzenesulfonyl)-N'-butylurea; N-(4-Methylphenylsulfonyl)-N'-butylurea; N-(p-Tolylsulfonyl)-N'-butylcarbamide; N-Butyl-N'-(4-methylphenylsulfonyl)urea; N-Butyl-N'-(p-tolylsulfonyl)urea; N-Butyl-N'-toluene-p-sulfonylurea; N-(p-tolylsulfonyl)-N'-n-butylurea; 1-Butyl-3-(4-methylphenylsulfonyl)urea; 1-Butyl-3-(p-methylphenylsulfonyl)urea; 1-Butyl-3-(p-tolylsulfonyl)urea; 1-Butyl-3-(para-tolylsulfonyl) urea; 1-Butyl-3-tosylurea; 1-butyl-3-(4-methylphenyl)sulfonylurea; 1-p-Toluenesulfonyl-3-butylurea; 3-(p-Tolyl-4-sulfonyl)-1-butylurea; 3-(p-tolylsulfonyl)-1-butylurea; 3-[p-Tolyl-4-sulfonyl]-1-butylurea
|
||||
| Drug Type |
Small molecular drug
|
||||
| Indication | Non-insulin dependent diabetes [ICD10:E11.9] | Approved | [551871] | ||
| Therapeutic Class |
Hypoglycemic Agents
|
||||
| Company |
Pharmacia And Upjohn Co
|
||||
| Structure |
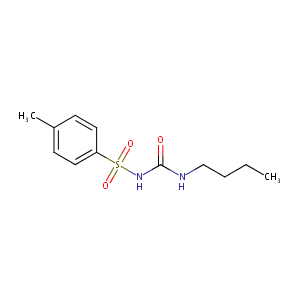
|
Download2D MOL |
|||
| Formula |
C12H18N2O3S
|
||||
| InChI |
InChI=1S/C12H18N2O3S/c1-3-4-9-13-12(15)14-18(16,17)11-7-5-10(2)6-8-11/h5-8H,3-4,9H2,1-2H3,(H2,13,14,15)
|
||||
| InChIKey |
JLRGJRBPOGGCBT-UHFFFAOYSA-N
|
||||
| CAS Number |
CAS 64-77-7
|
||||
| PubChem Compound ID | |||||
| PubChem Substance ID |
9357, 85591, 640044, 855782, 5658513, 7847446, 7980810, 8144417, 8149557, 8153385, 10321470, 10531694, 11111857, 11111858, 11120087, 11120575, 11121063, 11121572, 11122052, 11147170, 11335516, 11360755, 11362641, 11363341, 11364689, 11365203, 11365903, 11367251, 11367765, 11368465, 11369813, 11370553, 11370554, 11371678, 11372854, 11373366, 11373934, 11375413, 11375927, 11376627, 11377976, 11457019, 11461727, 11466218, 11467338, 11484736, 11485776, 11489026, 11490415, 11492173
|
||||
| ChEBI ID |
ChEBI:27999
|
||||
| SuperDrug ATC ID |
A10BB03
|
||||
| SuperDrug CAS ID |
cas=000064777
|
||||
| Target and Pathway | |||||
| Target(s) | SUR2-type K(ATP) channel | Target Info | Blocker | [536969] | |
| Sulfonylurea receptor 1 | Target Info | Blocker | [536969] | ||
| Pathway Interaction Database | FOXA2 and FOXA3 transcription factor networks | ||||
| PathWhiz Pathway | Muscle/Heart Contraction | ||||
| Pancreas Function | |||||
| WikiPathways | Potassium Channels | ||||
| Integration of energy metabolism | |||||
| References | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

