Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D0J9HW
|
||||
| Former ID |
DCL000583
|
||||
| Drug Name |
Olaparib
|
||||
| Synonyms |
AZD 2281; AZD2281; AZD-2281; Acylpiperazine analogue, 47; KU-0059436; KU-59436; Olaparib, KU-0059436, AZD2281,KU0059436, AZD2281; 4-[(3-{[4-Cyclopropylcarbonyl)piperazin-4-yl]carbonyl}-4-fluorophenyl)methyl]phtalazin-1(2H)-one; 4-[3-(4-Cyclopropanecarbonyl-piperazine-1-carbonyl)-4-fluoro-benzyl]-2H-phthalazin-1-one
|
||||
| Drug Type |
Small molecular drug
|
||||
| Company |
AstraZeneca
|
||||
| Structure |
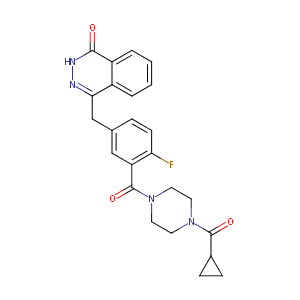
|
Download2D MOL |
|||
| Formula |
C24H23FN4O3
|
||||
| InChI |
InChI=1S/C24H23FN4O3/c25-20-8-5-15(14-21-17-3-1-2-4-18(17)22(30)27-26-21)13-19(20)24(32)29-11-9-28(10-12-29)23(31)16-6-7-16/h1-5,8,13,16H,6-7,9-12,14H2,(H,27,30)
|
||||
| InChIKey |
FDLYAMZZIXQODN-UHFFFAOYSA-N
|
||||
| CAS Number |
CAS 763113-22-0
|
||||
| PubChem Compound ID | |||||
| PubChem Substance ID |
47209066, 56053773, 57299275, 85197660, 93581006, 99436961, 103605183, 109692964, 117670453, 118049496, 121277945, 123051082, 124490470, 124756970, 124947876, 125163775, 125312522, 125415525, 126582065, 126626885, 126644879, 126664211, 126666996, 126738835, 131407195, 131465127, 134213960, 134222742, 134338825, 134339140, 134346179, 134964396, 135261055, 135697767, 135708109, 135723596, 135727477, 136368068, 136377786, 136895641, 136920280, 137005640, 137255348, 141853509, 143499369, 144115666, 152237700, 152258100, 152344243, 160646939
|
||||
| Target and Pathway | |||||
| Target(s) | Poly ADP ribose polymerase (PARP) | Target Info | Modulator | [533123] | |
| References | |||||
| Ref 542530 | (http://www.guidetopharmacology.org/) Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7519). | ||||
| Ref 550296 | LYNPARZA? approved by the US food and drug administration for the treatment of advanced ovarian cancer in patients with germline BRCA-mutations | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

