Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D0NR6S
|
||||
| Former ID |
DAP000308
|
||||
| Drug Name |
Maraviroc
|
||||
| Synonyms |
Celsentri; MVC; Selzentry; Maraviroc [USAN]; UK 427857; Celsentri (TN); Celsentri(TM); PRO 140 & Maraviroc; Selzentry (TN); Selzentry(TM); UK-427857; UK-427,857; UK-427,857 maraviroc (MVC); Exo-4,4-Difluoro-N-[3-[3-(3-isopropyl-5-methyl-4H-1,2,4-triazol-4-yl)-8-azabicyclo[3.2.1]oct-8-yl]-1(S)-phenylpropyl]cyclohexanecarboxamide; PRO 140 (Anti-CCR5 monoclonal antibody) & exo-4,4-Difluoro-N-[3-[3-(3-isopropyl-5-methyl-4H-1,2,4-triazol-4-yl)-8-azabicyclo[3.2.1]oct-8-yl]-1(S)-phenylpropyl]cyclohexanecarboxamide; Isopropyl, 4,4-difluoro-N-((1S)-3-{(1R,3s,5S)-3-(3-methyl-5-(propan-2-yl)-4H-1,2,4-triazol-4-yl)-8-azabicyclo(3.2.1)octan-8-yl}-1-phenylpropyl)cyclohexanecarboxamide; 4,4-Difluoro-N-((1S)-3-(exo-3-(3-isopropyl-5-methyl-4H-1,2,4-triazol-4-yl)-8-azabicyclo(3.2.1)oct-8-yl)-1-phenylpropyl)cyclohexanecarboxamide; 4,4-difluoro-N-[(1S)-3-[(1R,5S)-3-(3-methyl-5-propan-2-yl-1,2,4-triazol-4-yl)-8-azabicyclo[3.2.1]octan-8-yl]-1-phenylpropyl]cyclohexane-1-carboxamide
|
||||
| Drug Type |
Small molecular drug
|
||||
| Therapeutic Class |
Anti-HIV Agents
|
||||
| Company |
Pfizer; AstraZeneca
|
||||
| Structure |
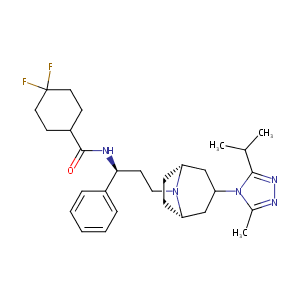
|
Download2D MOL |
|||
| Formula |
C29H41F2N5O
|
||||
| InChI |
InChI=1S/C29H41F2N5O/c1-19(2)27-34-33-20(3)36(27)25-17-23-9-10-24(18-25)35(23)16-13-26(21-7-5-4-6-8-21)32-28(37)22-11-14-29(30,31)15-12-22/h4-8,19,22-26H,9-18H2,1-3H3,(H,32,37)/t23-,24+,25?,26-/m0/s1
|
||||
| InChIKey |
GSNHKUDZZFZSJB-HLMSNRGBSA-N
|
||||
| CAS Number |
CAS 376348-65-1
|
||||
| PubChem Compound ID | |||||
| PubChem Substance ID |
642129, 12015618, 16284914, 24721602, 26602278, 34668114, 34939567, 46508040, 50066636, 50681186, 57410169, 92719325, 103771170, 104055495, 104179071, 111611845, 124894309, 124894310, 125085088, 126620904, 126649000, 126667024, 134337620, 134340611, 135246807, 135611105, 135650567, 135651174, 135692886, 135723692, 136340231, 137006237, 137255791, 137255792, 137434072, 143497951, 152164500, 152238539, 152258411, 152344350, 160647248, 160967811, 162201744, 162222523, 162894542, 164027255, 164339310, 164828285, 174529188, 175265823
|
||||
| SuperDrug ATC ID |
J05AX09
|
||||
| Drug Resistance Mutation (DRM) | |||||
| DRM | DRM Info | ||||
| Target and Pathway | |||||
| Target(s) | C-C chemokinereceptor type 5 | Target Info | Antagonist | [534846], [537430], [537962] | |
| Pathway Interaction Database | IL12-mediated signaling events | ||||
| References | |||||
| Ref 529282 | 2007 FDA drug approvals: a year of flux. Nat Rev Drug Discov. 2008 Feb;7(2):107-9. | ||||
| Ref 536223 | Emerging drugs for the treatment of chronic obstructive pulmonary disease. Expert Opin Emerg Drugs. 2006 May;11(2):275-91. | ||||
| Ref 542947 | (http://www.guidetopharmacology.org/) Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 806). | ||||
| Ref 534846 | Tyrosine sulfation of the amino terminus of CCR5 facilitates HIV-1 entry. Cell. 1999 Mar 5;96(5):667-76. | ||||
| Ref 537430 | Rapamycin enhances aplaviroc anti-HIV activity: implications for the clinical development of novel CCR5 antagonists. Antiviral Res. 2009 Jul;83(1):86-9. Epub 2009 Mar 9. | ||||
| Ref 537962 | Molecular cloning and functional expression of a new human CC-chemokine receptor gene. Biochemistry. 1996 Mar 19;35(11):3362-7. | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

